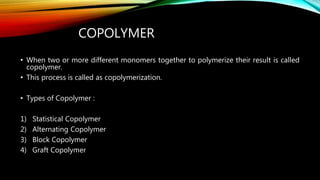
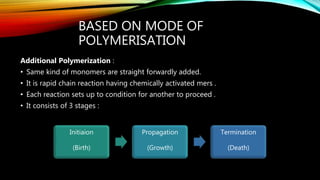
Polymers are large molecules formed from repeating structural units called monomers. There are several types of polymers including natural polymers like DNA, homopolymers made of identical monomers, and copolymers made of two or more different monomers. Polymers can also be classified based on their structure, molecular forces, and mode of polymerization. They have a variety of applications in medicine, consumer products, industry, and sports due to their properties like low density, corrosion resistance, and ability to be produced in different colors.