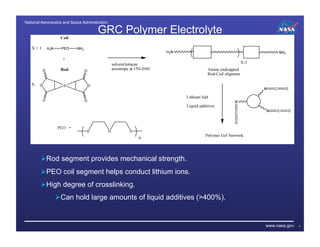
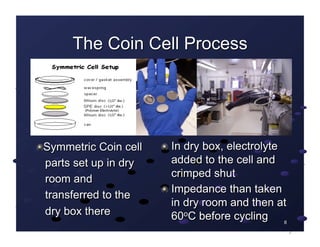
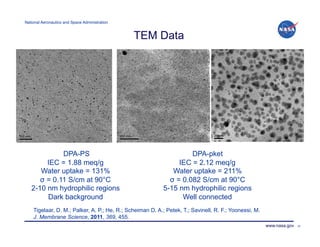
The document discusses recent advancements in lithium batteries and fuel cells by the NASA Glenn Research Center, emphasizing the development of polymer/ionic liquid electrolytes for lithium batteries with improved conductivity and stability. Key findings highlight that specific combinations of ionic liquid and lithium salt concentrations significantly enhance cycling stability and compatibility with lithium electrodes. Additionally, it outlines the synthesis of novel proton exchange membranes for fuel cells, focusing on their high thermal stability and potential applications in various energy systems.