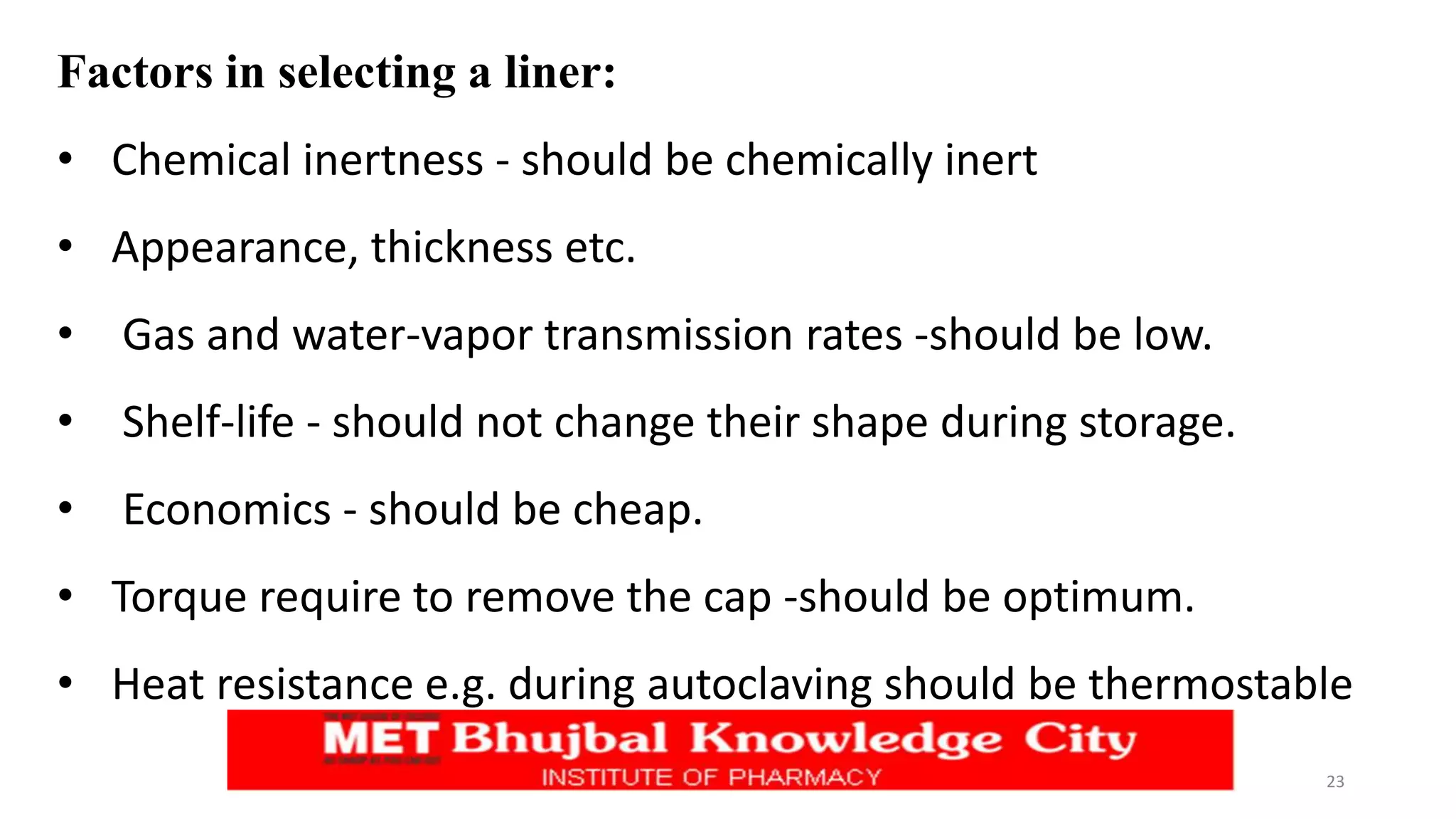
This document discusses types of closures and closure liners used for containers. It describes five basic closure designs: screw on, crimp on, press on, roll on, and friction fit. It also discusses tamperproof, child resistant, and dispenser applicator variations. Common closure materials are plastic, metal, and laminates. Closure liners are used to create a seal and come in homogeneous one-piece or heterogeneous multi-layer designs. Selection factors for closures and liners include chemical inertness, permeability, stability, and economics. Regulations require packaging materials to preserve drug quality and safety.