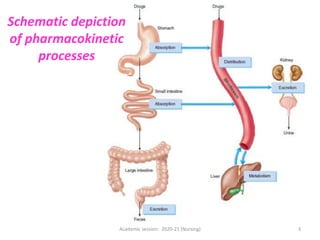
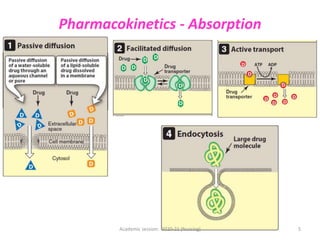
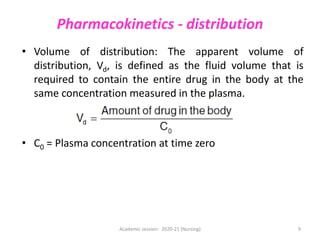
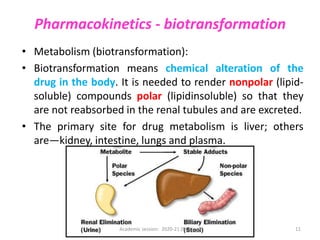
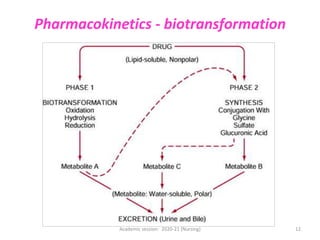
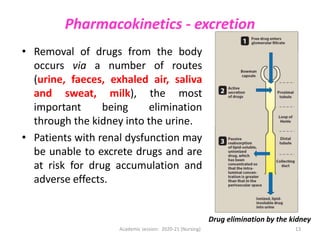
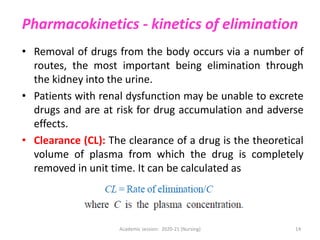
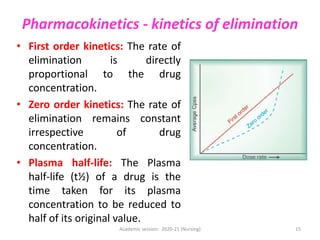
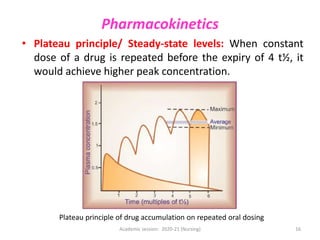
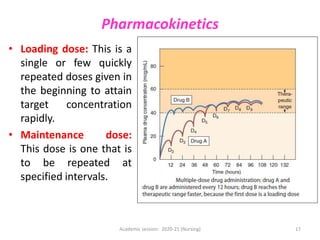
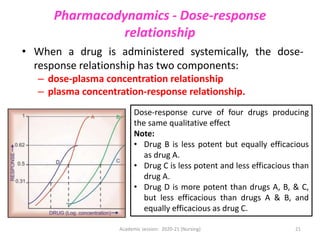
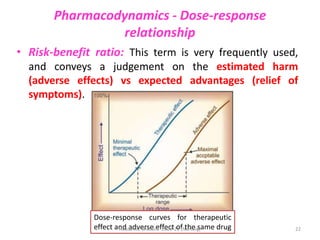
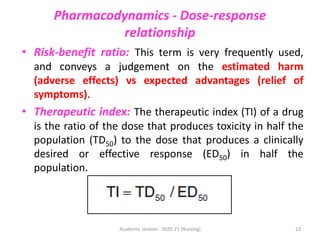
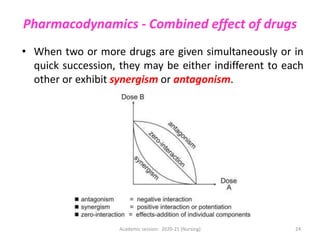
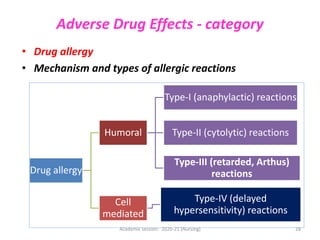
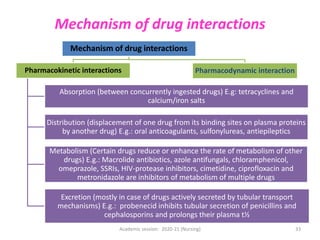
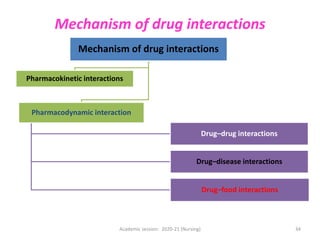
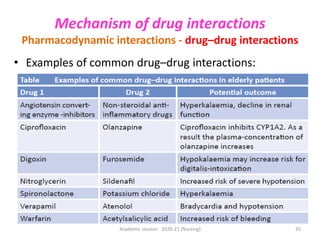
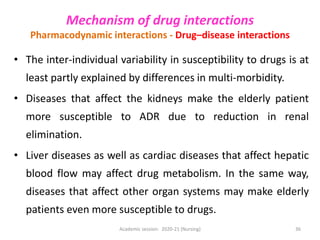
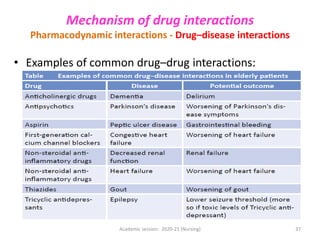
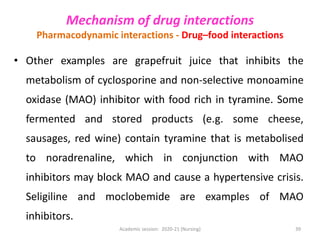
The document discusses pharmacokinetics and pharmacodynamics. It describes the four main processes of pharmacokinetics - absorption, distribution, metabolism and elimination - and how they determine the effects of drugs in the body. It also discusses factors that influence absorption and distribution of drugs. The document then covers pharmacodynamics concepts such as dose-response relationships, therapeutic indices, and interactions between drugs. Mechanisms of drug interactions including pharmacokinetic and pharmacodynamic interactions are explained. Common types of drug interactions and ways to prevent adverse drug effects are also summarized.