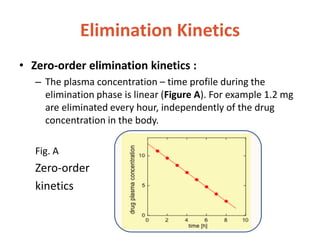
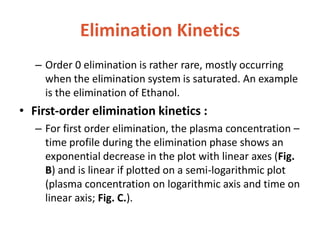
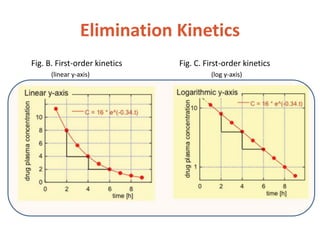
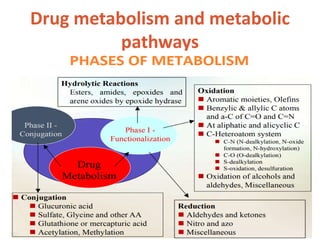
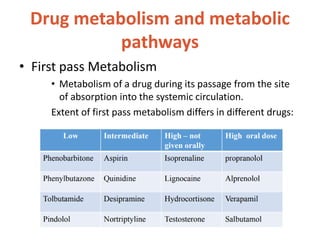
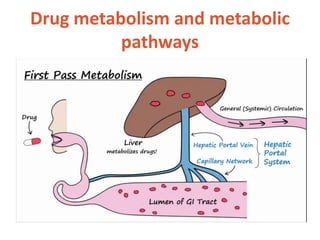
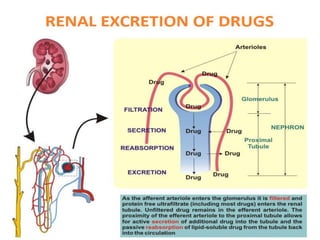
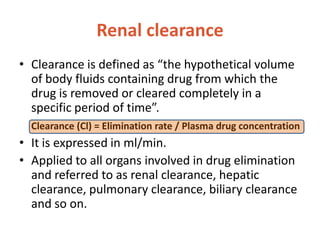
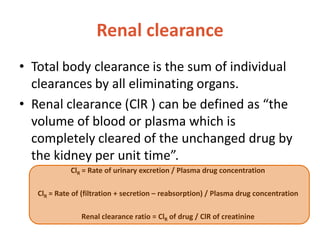
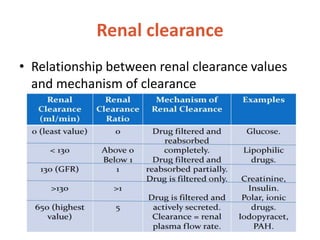
This document discusses drug elimination, which involves biotransformation (metabolism) and excretion of drugs from the body. It describes zero-order and first-order elimination kinetics, drug metabolism pathways including phase I and II reactions, and factors that influence renal excretion of drugs such as physicochemical properties and plasma concentration. Renal clearance is defined as the volume of plasma cleared of drug per unit time by the kidneys. Non-renal routes of excretion include biliary, pulmonary, dermal and gastrointestinal excretion.