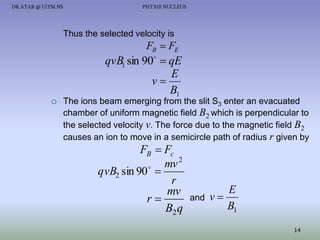
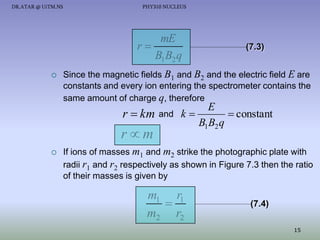
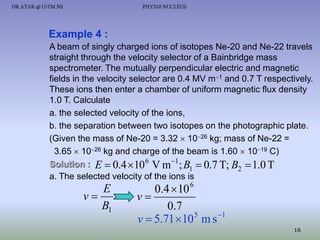
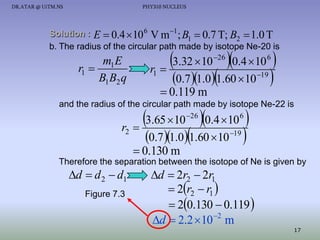
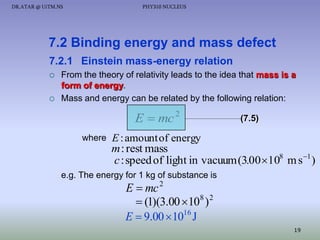
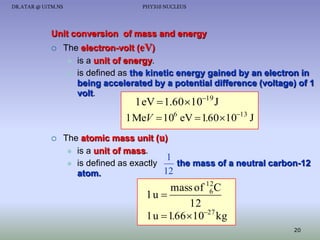
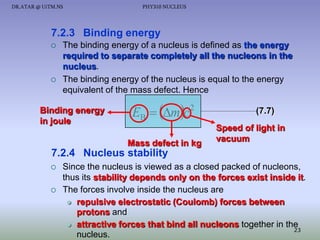
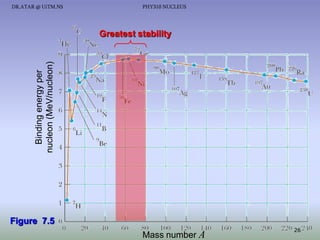
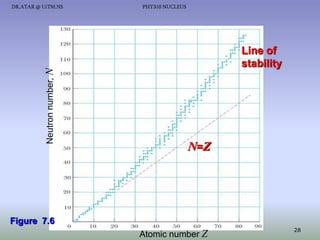
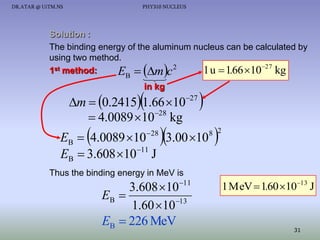
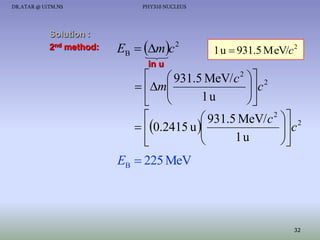
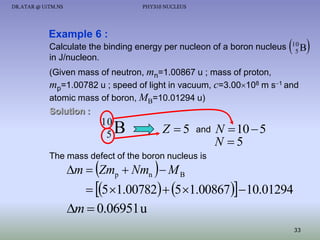
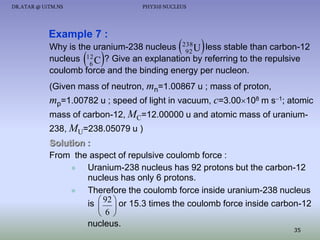
The document discusses properties of the nucleus, including:
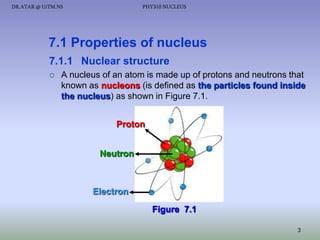
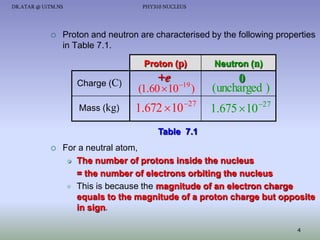
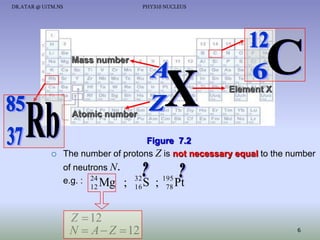
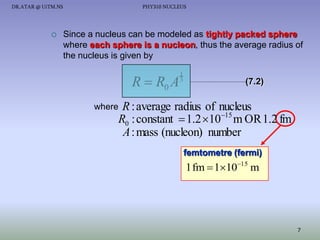
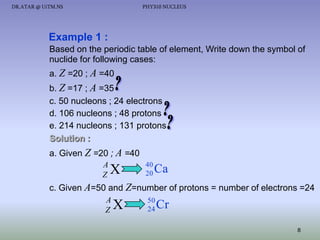
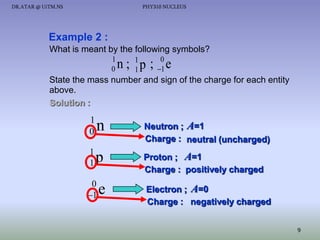
- The nucleus contains positively charged protons and uncharged neutrons.
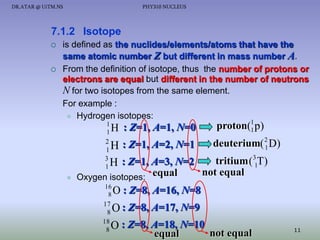
- Isotopes of an element have the same number of protons but different numbers of neutrons.
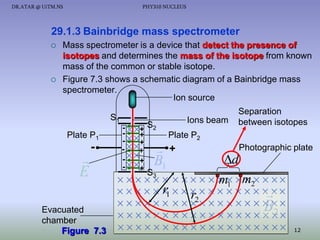
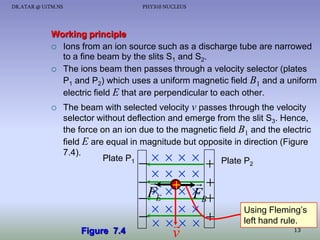
- A mass spectrometer can be used to separate isotopes based on their different masses, producing a characteristic pattern on a photographic plate.