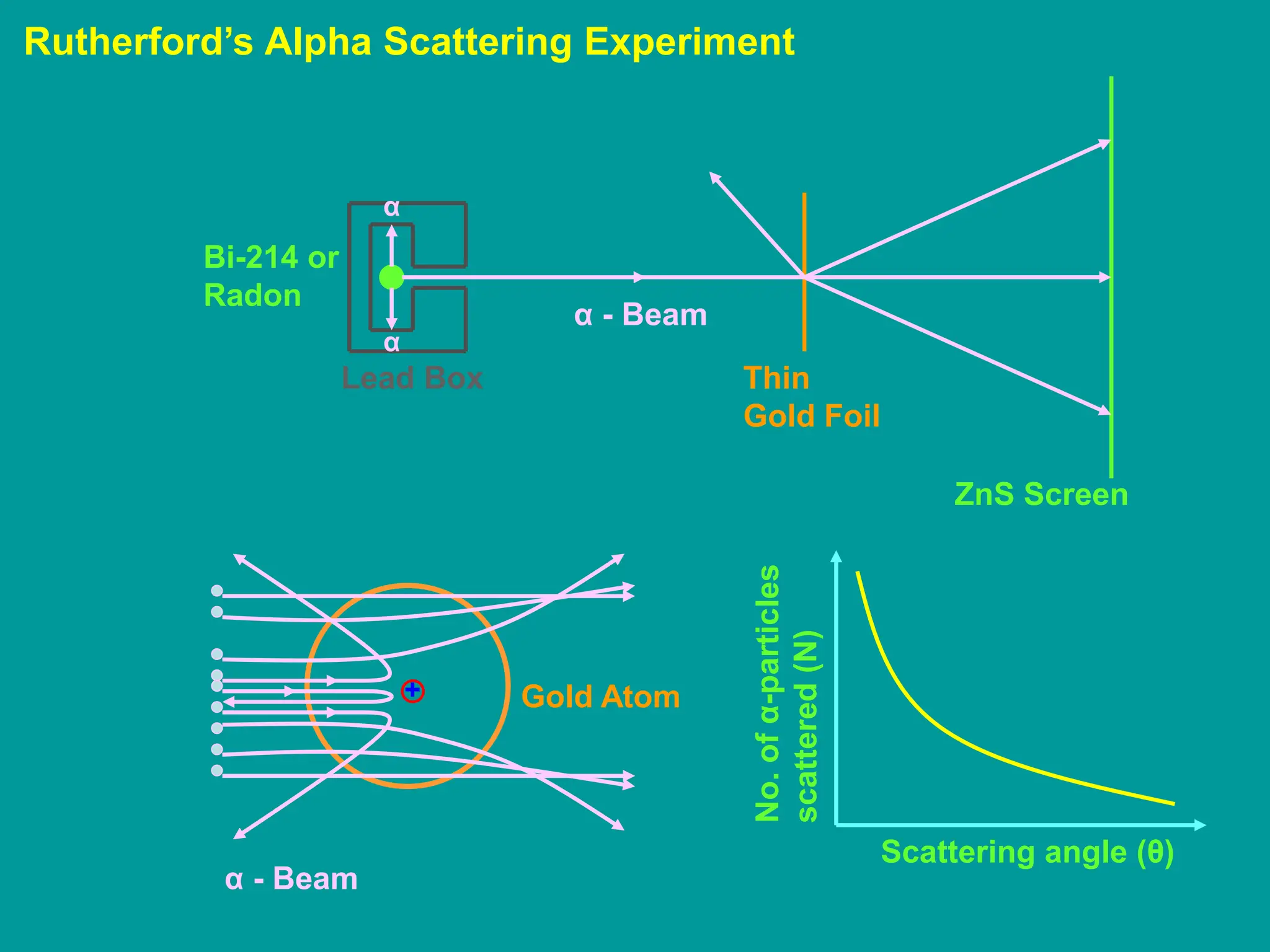
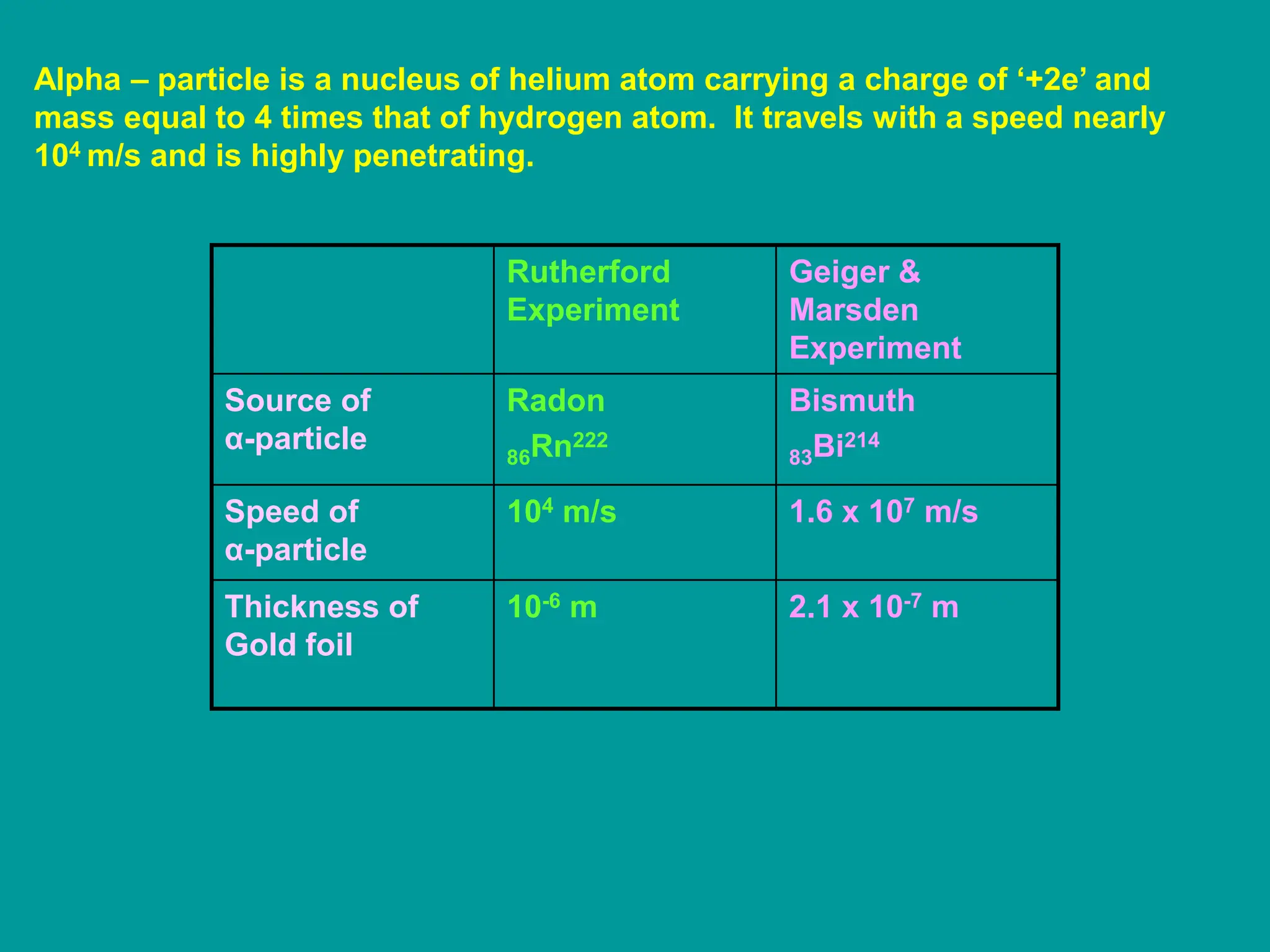
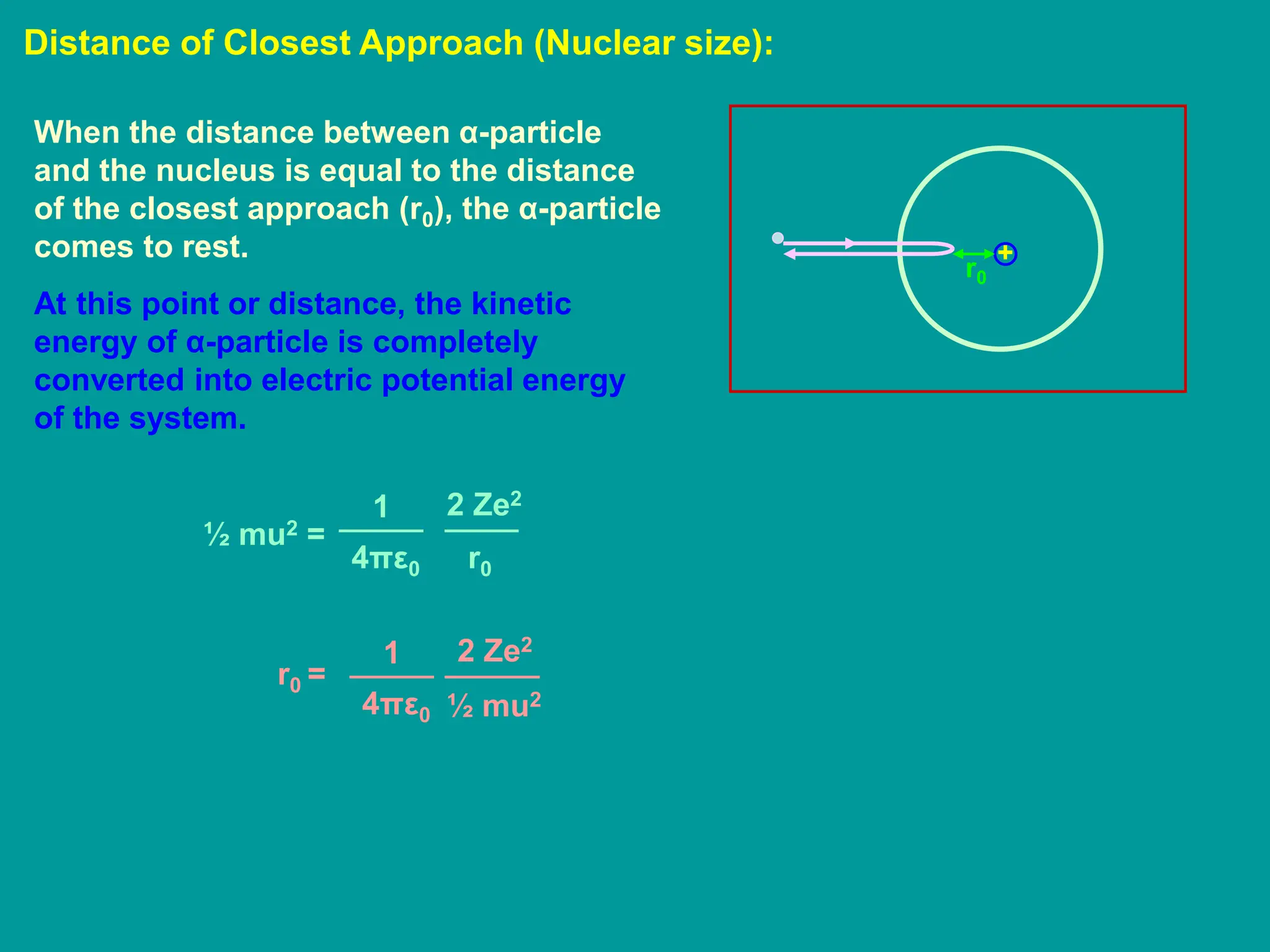
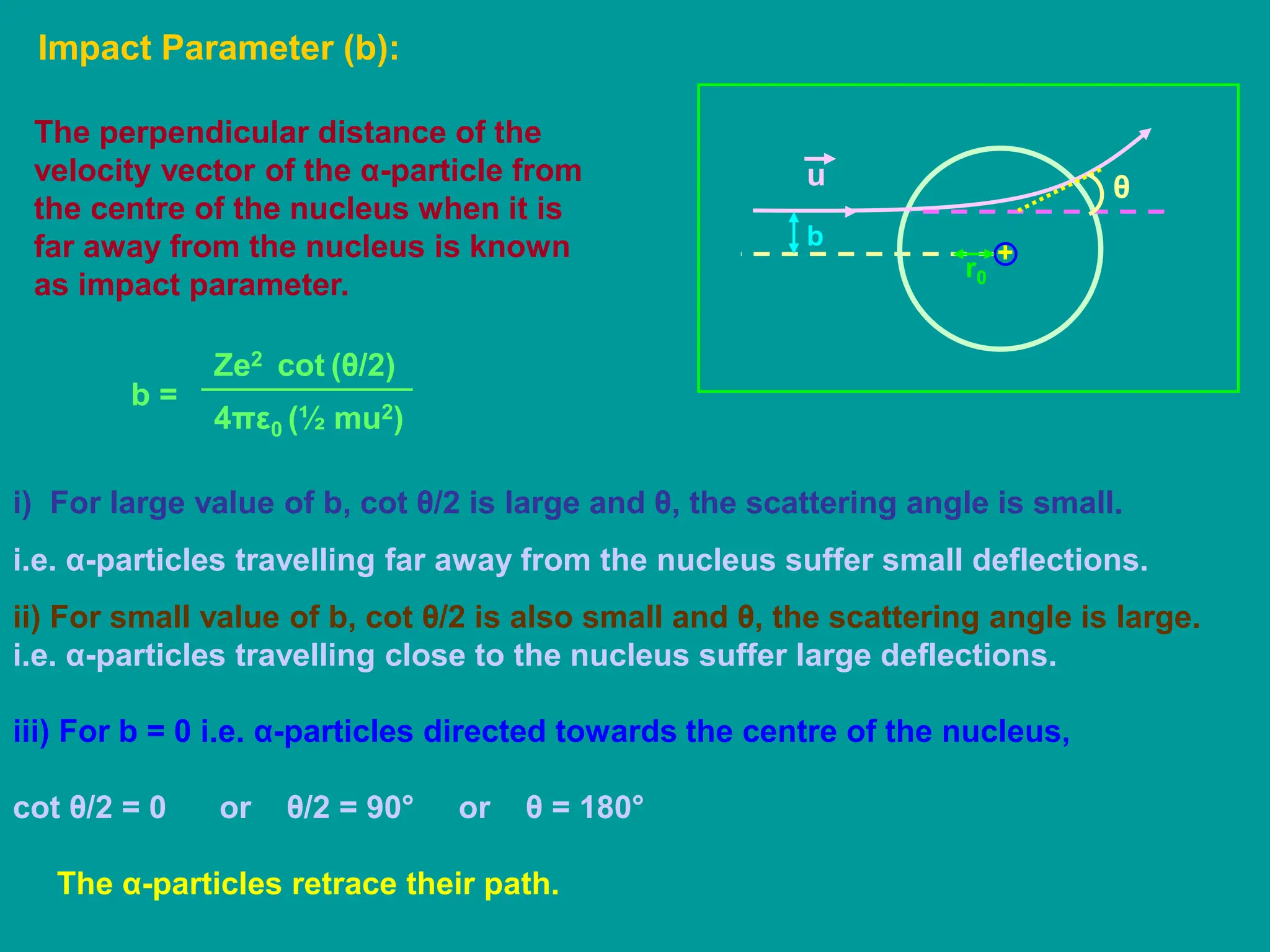
1. Rutherford's alpha scattering experiment showed that the positive charge and mass of an atom are concentrated in a tiny nucleus at the center. Some alpha particles were deflected through large angles, including backwards, indicating the presence of a dense, positively charged nucleus.
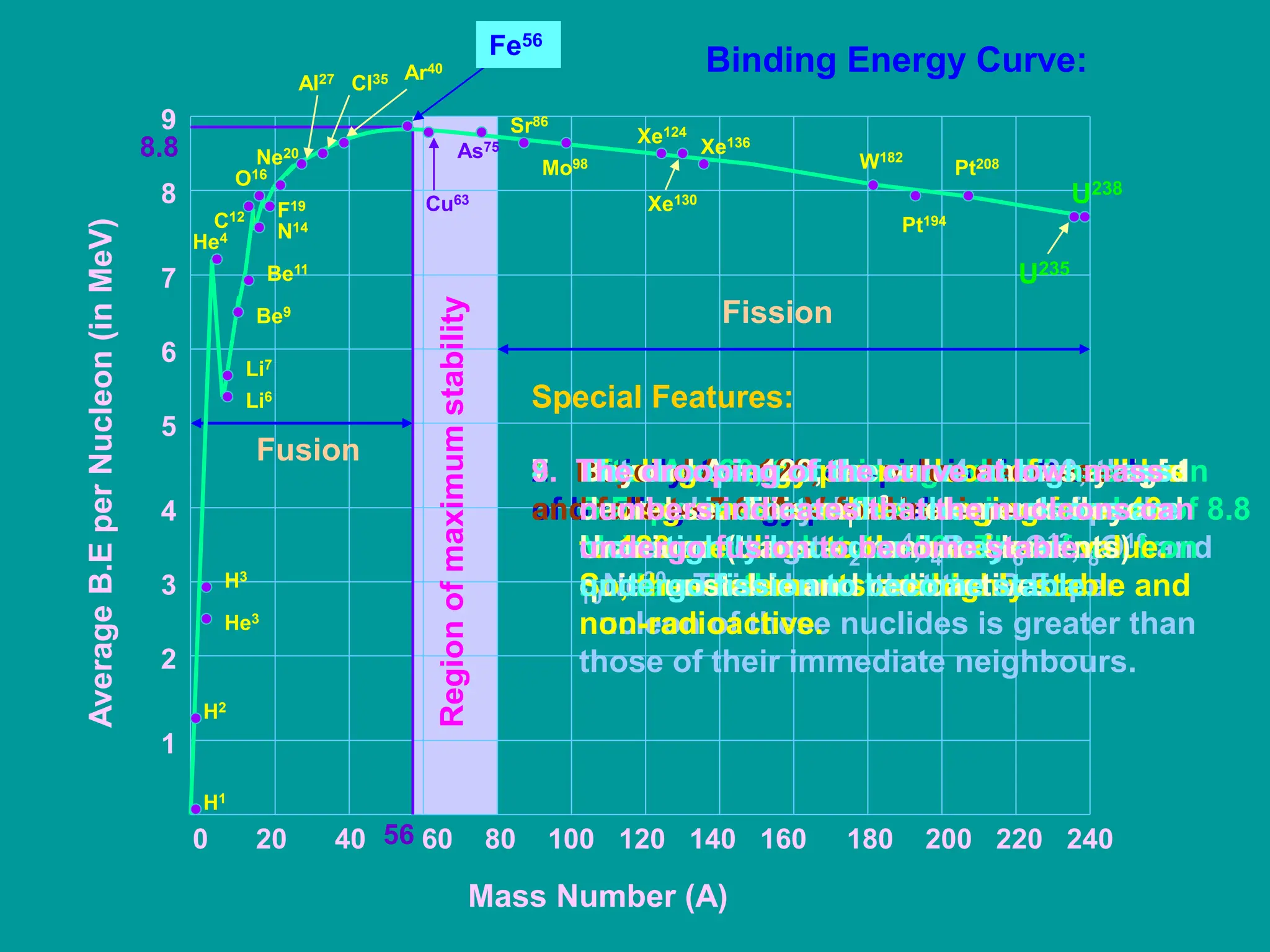
2. The binding energy curve shows that binding energy per nucleon increases initially with mass number, peaks at iron-56, then decreases, making very large and very small nuclei unstable. Nuclides with mass numbers from 40-120 have binding energies close to the maximum, making them highly stable.
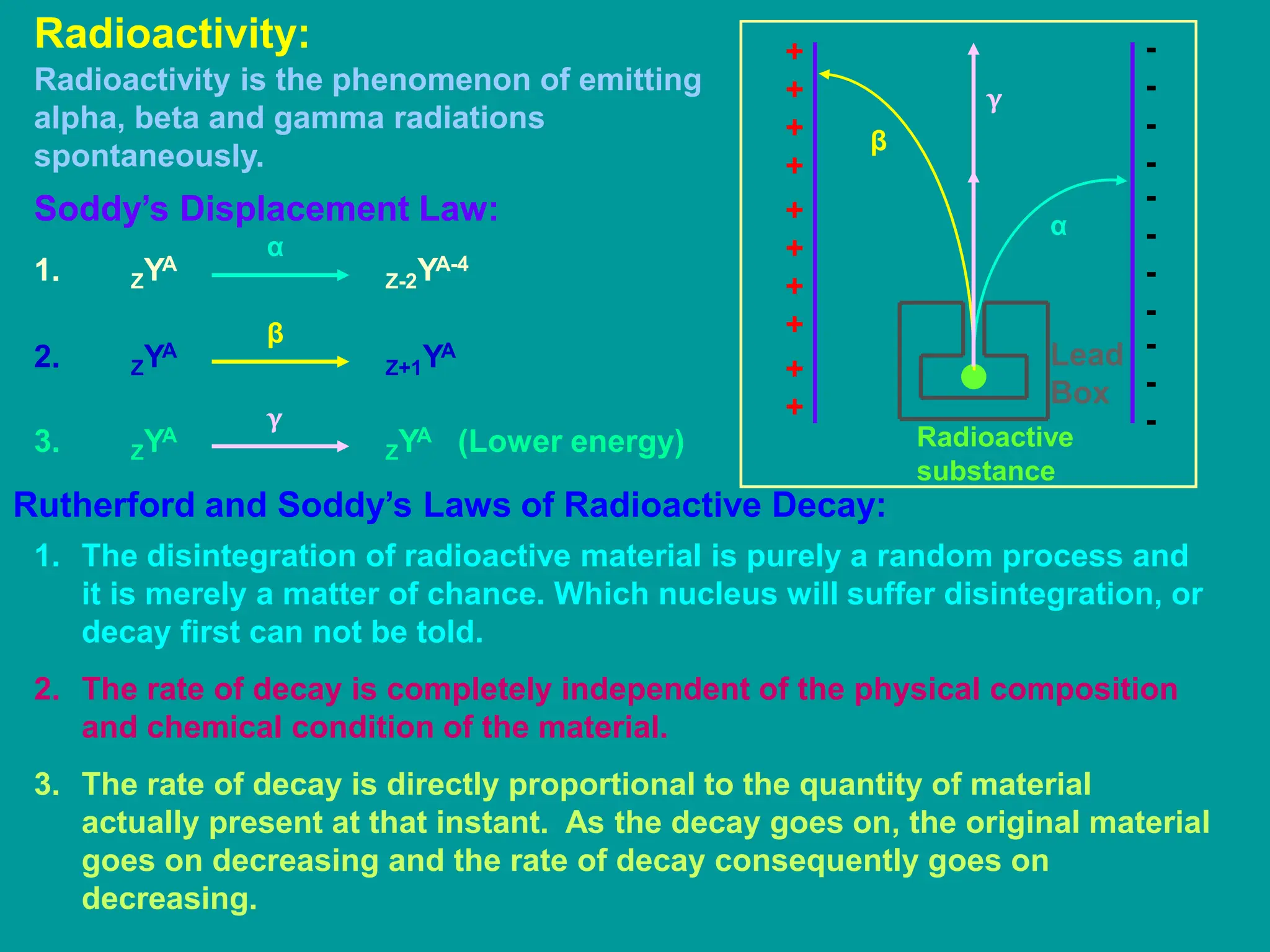
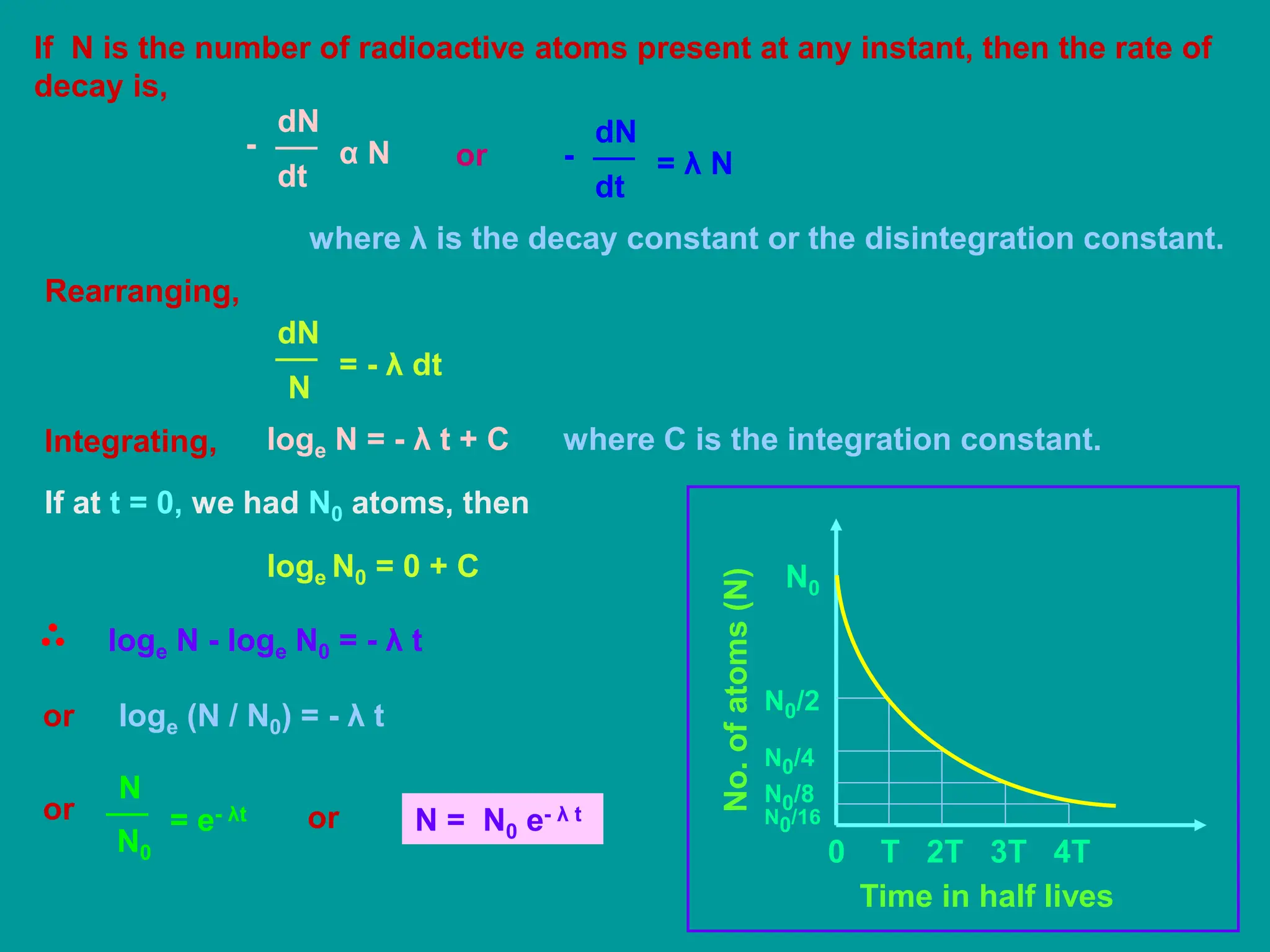
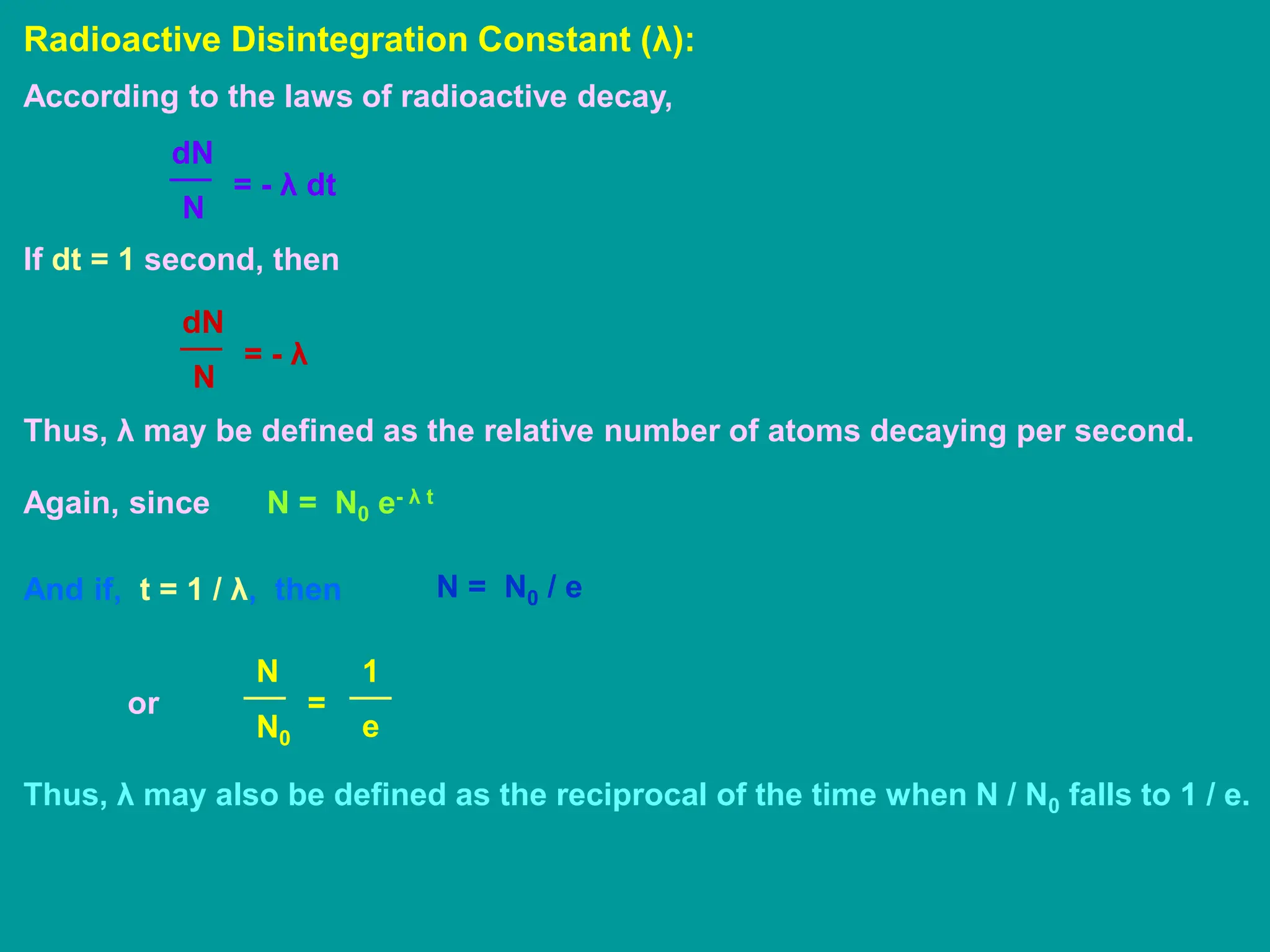
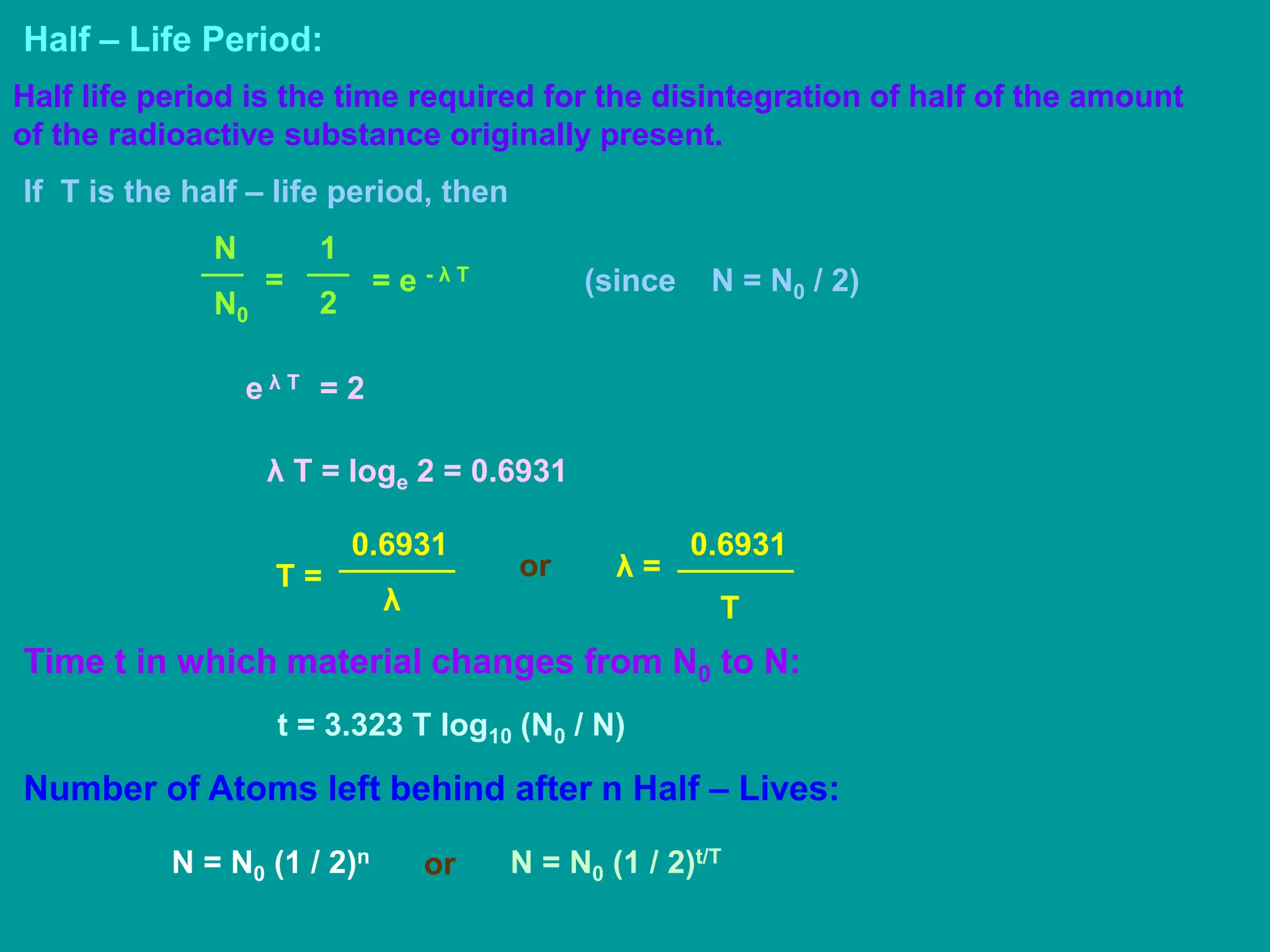
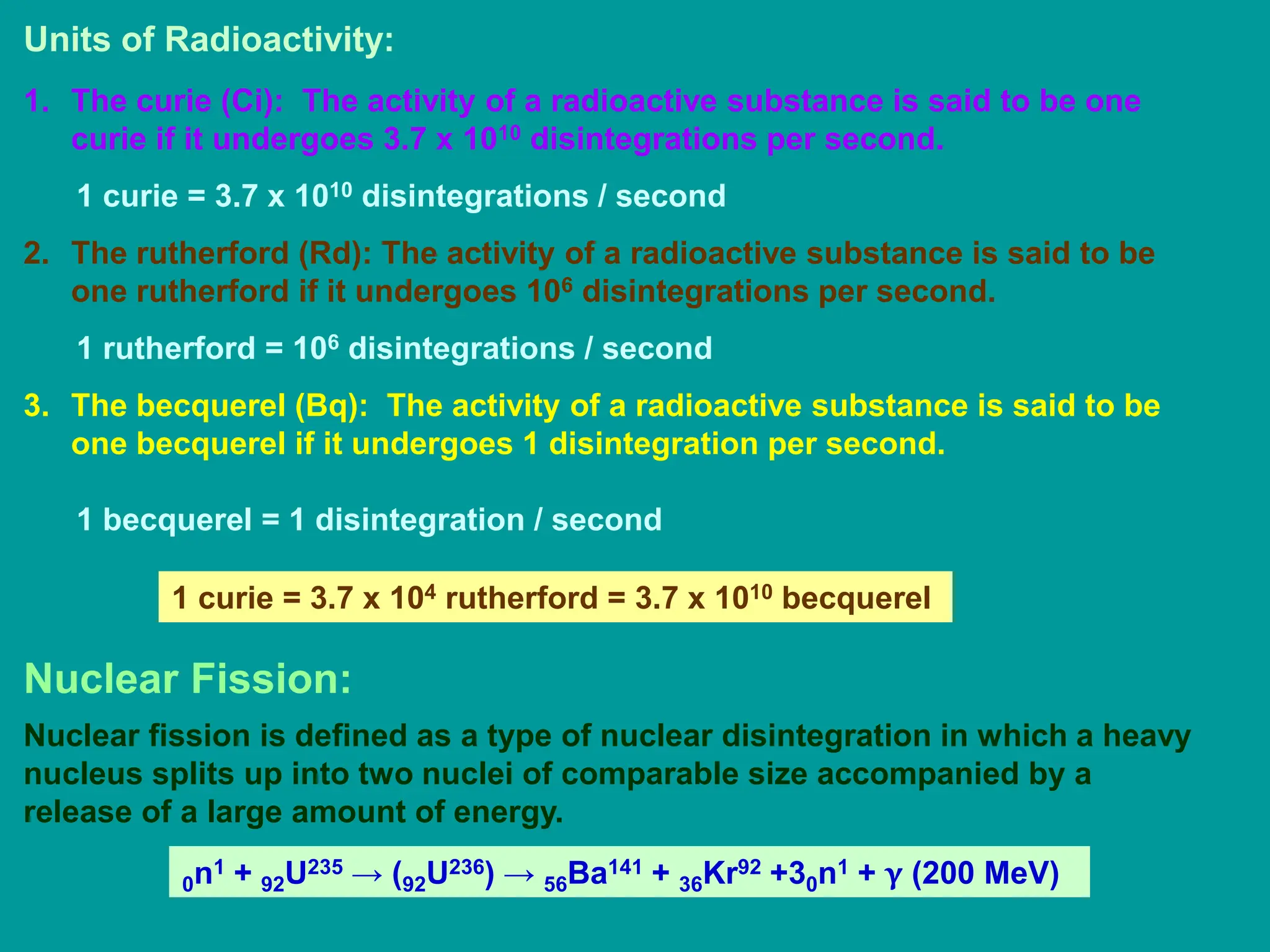
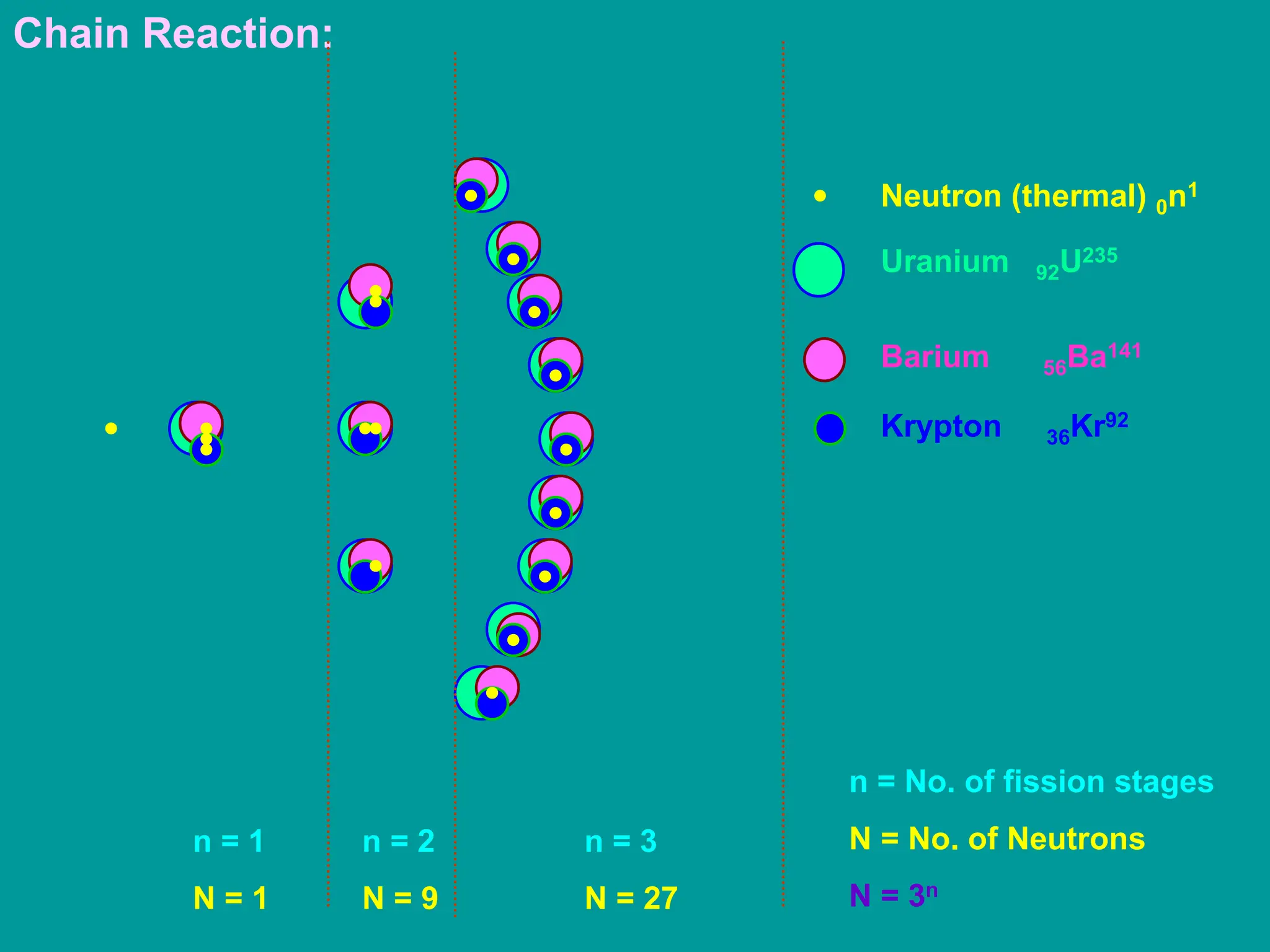
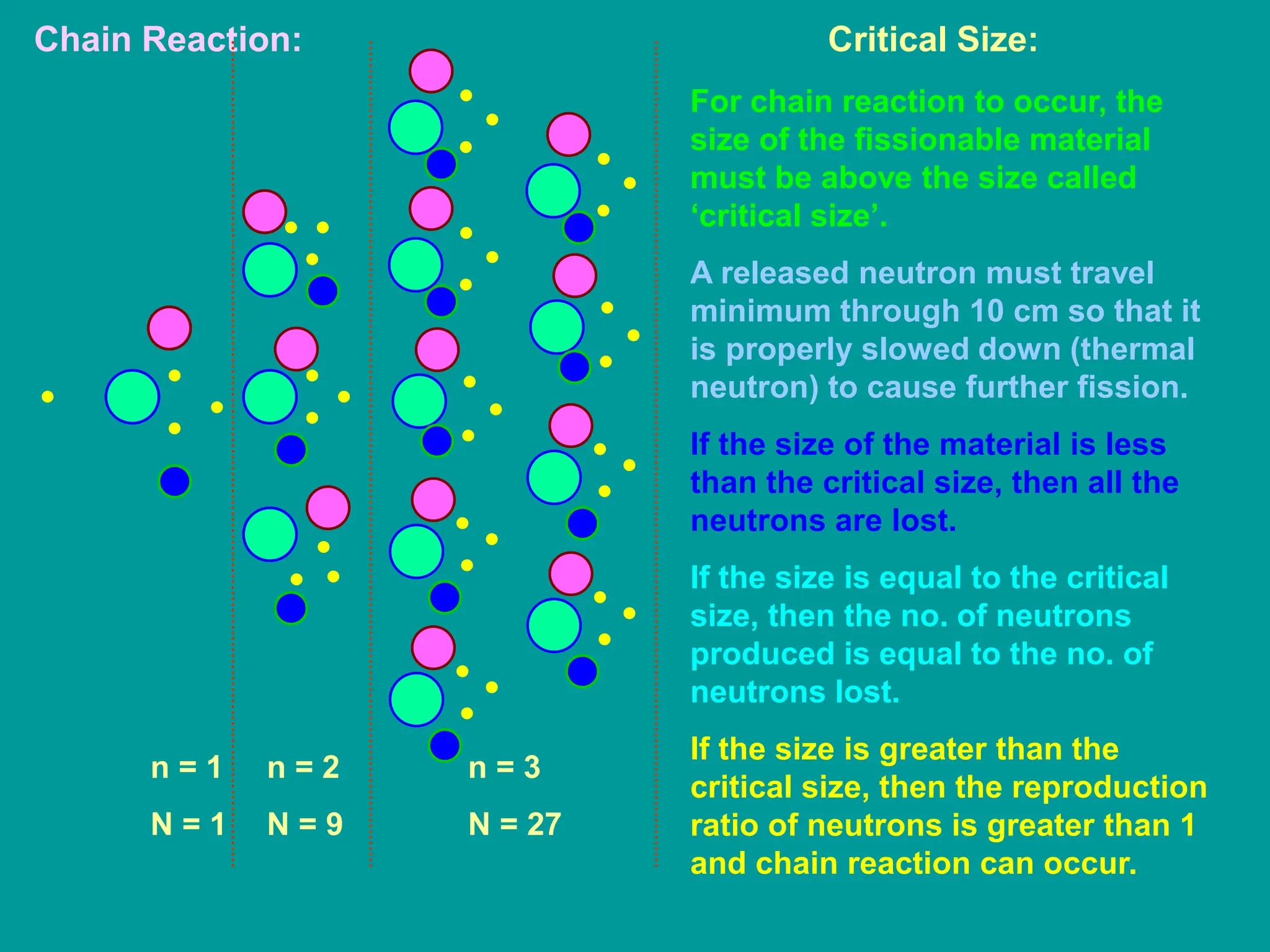
3. Radioactive decay occurs spontaneously via emission of alpha, beta, or gamma radiation. The rate of decay is proportional to the amount of radioactive material and



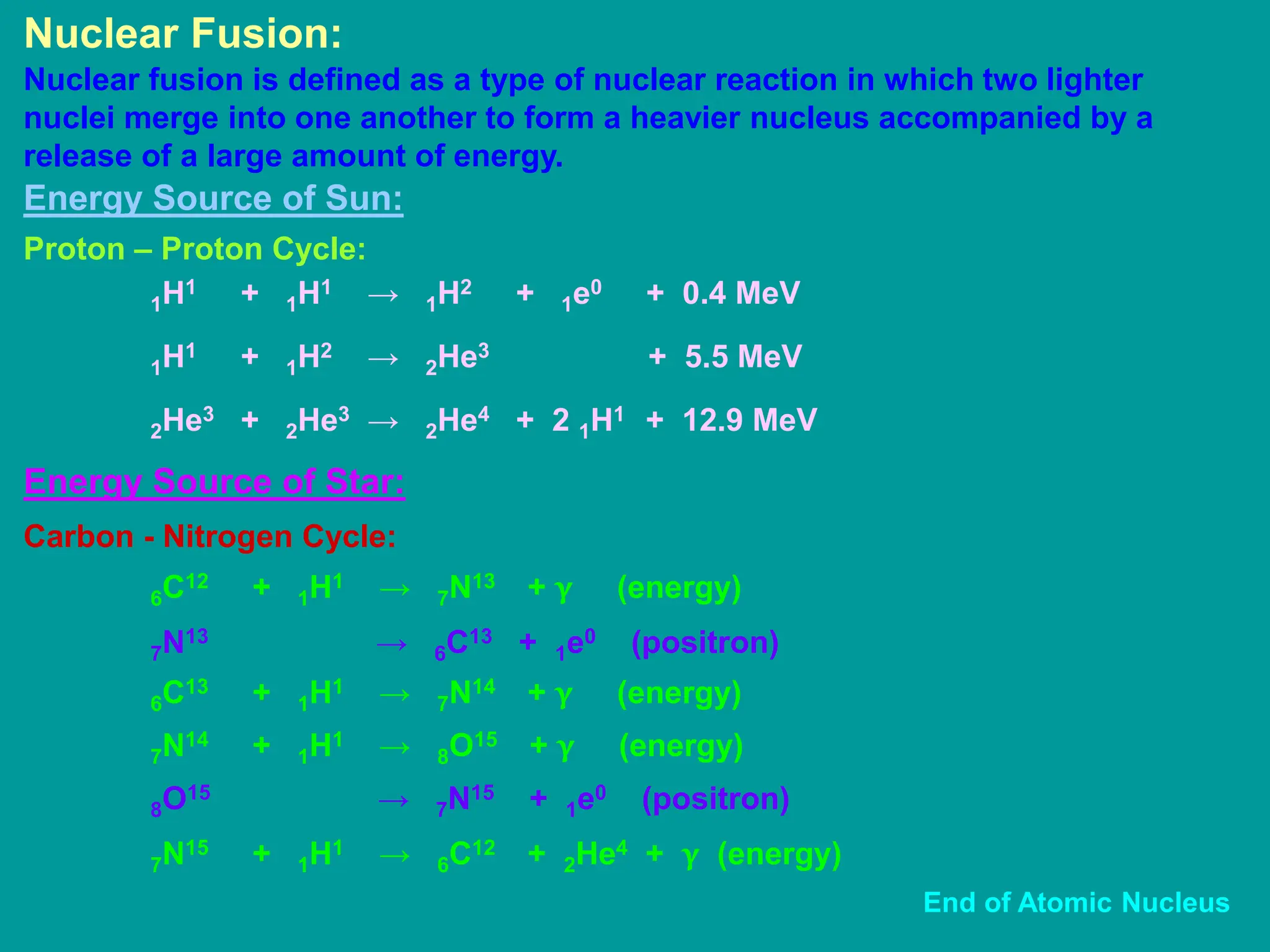
![Mass – Energy Relation:
According to Newton’s second law of motion, force acting on a body is
defined as the rate of change of momentum.
d
dt
F = (mv)
dv
dt
= m
dm
dt
+ v
If this force F displaces the body by a distance dx, its energy increases by
dv
dt
= m
dK = F.dx dx
dm
dt
+ v dx
dx
dt
= m
dK dv
dx
dt
+ v dm
= m v dv + v2 dm ………… (1)
dK
According to Einstein’s relation of relativistic mass,
m =
m0
[1 – (v2 / c2)]½](https://image.slidesharecdn.com/atomicnucleus-240227195152-fcf9ff37/75/Atomic_Nucleus-ppt-for-general-physics-2-10-2048.jpg)
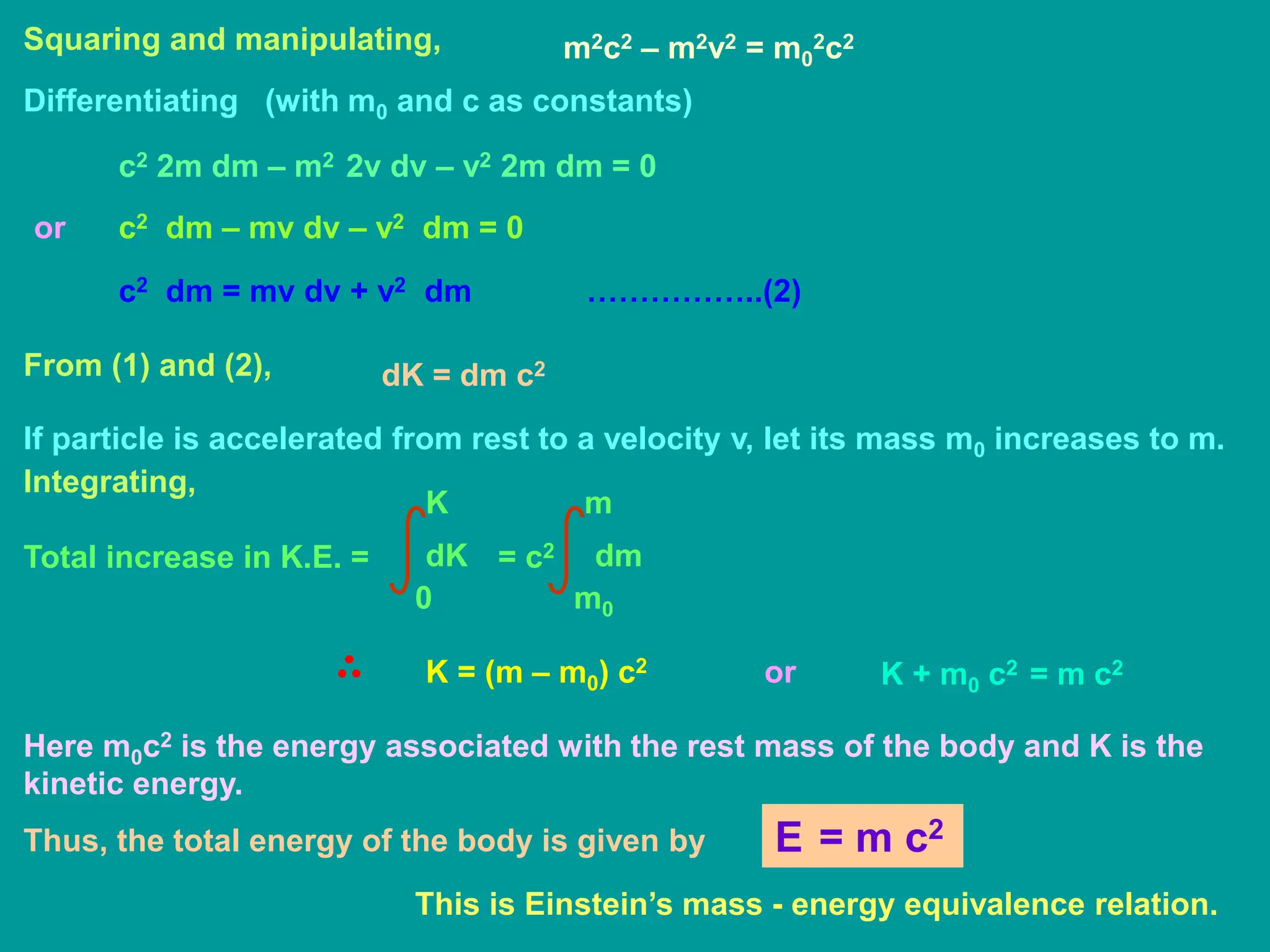
![Mass Defect:
It is the difference between the rest mass of the nucleus and the sum of the
masses of the nucleons composing a nucleus is known as mass defect.
Δm = [ Zmp + (A – Z) mn ] - M
Mass defect per nucleon is called packing fraction.
Binding Energy:
It is the energy required to break up a nucleus into its constituent parts and
place them at an infinite distance from one another.
B.E = Δm c2
Nuclear Forces:
They are the forces between p – p, p – n or n – n in the nucleus. They can be
explained by Meson Theory.
There are three kinds of mesons – positive (π+), negative (π-) and neutral (π0).
π+ and π- are 273 times heavier than an electron.
π0 is 264 times heavier than an electron.
Nucleons (protons and neutrons) are surrounded by mesons.](https://image.slidesharecdn.com/atomicnucleus-240227195152-fcf9ff37/75/Atomic_Nucleus-ppt-for-general-physics-2-12-2048.jpg)