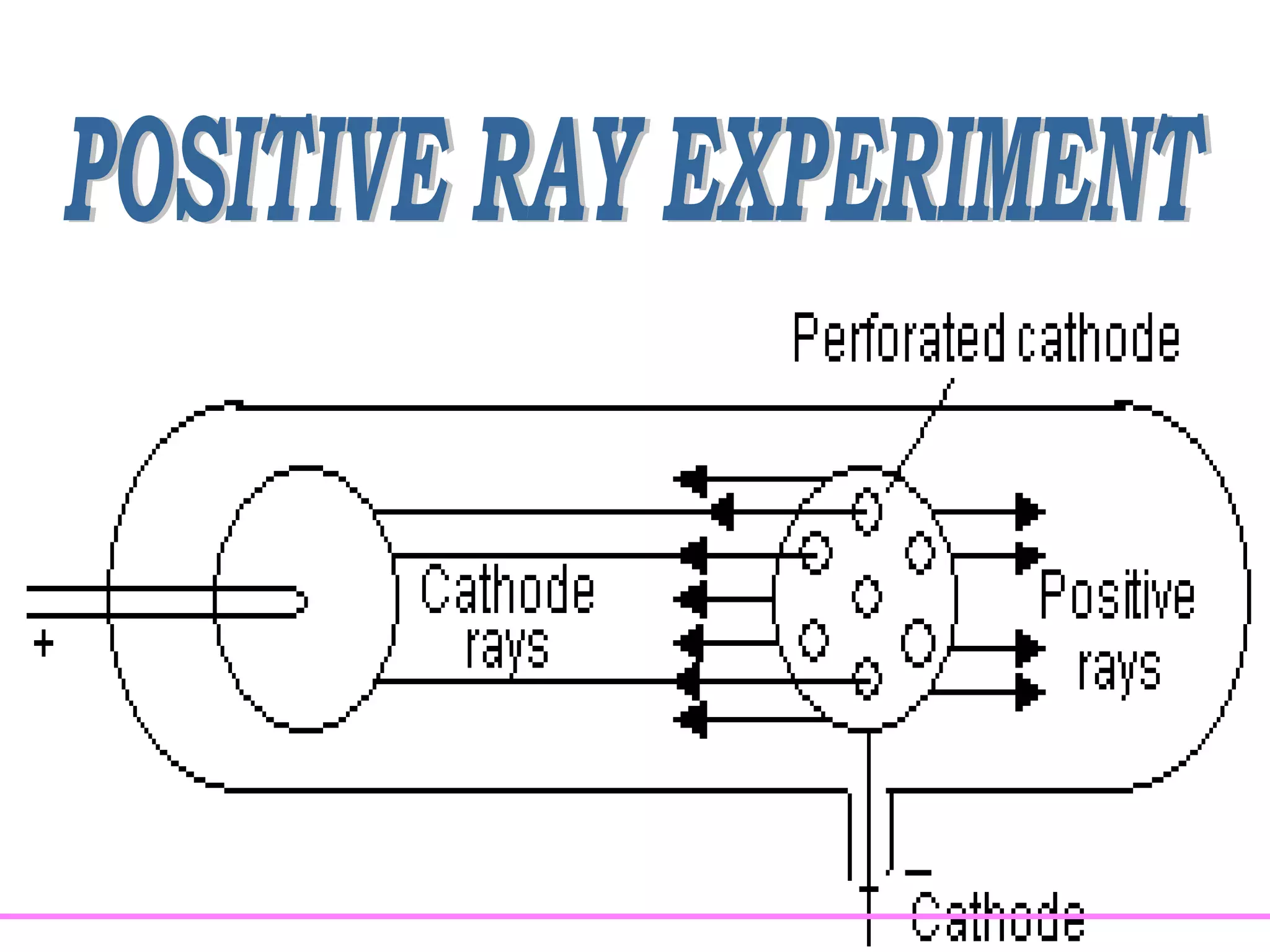
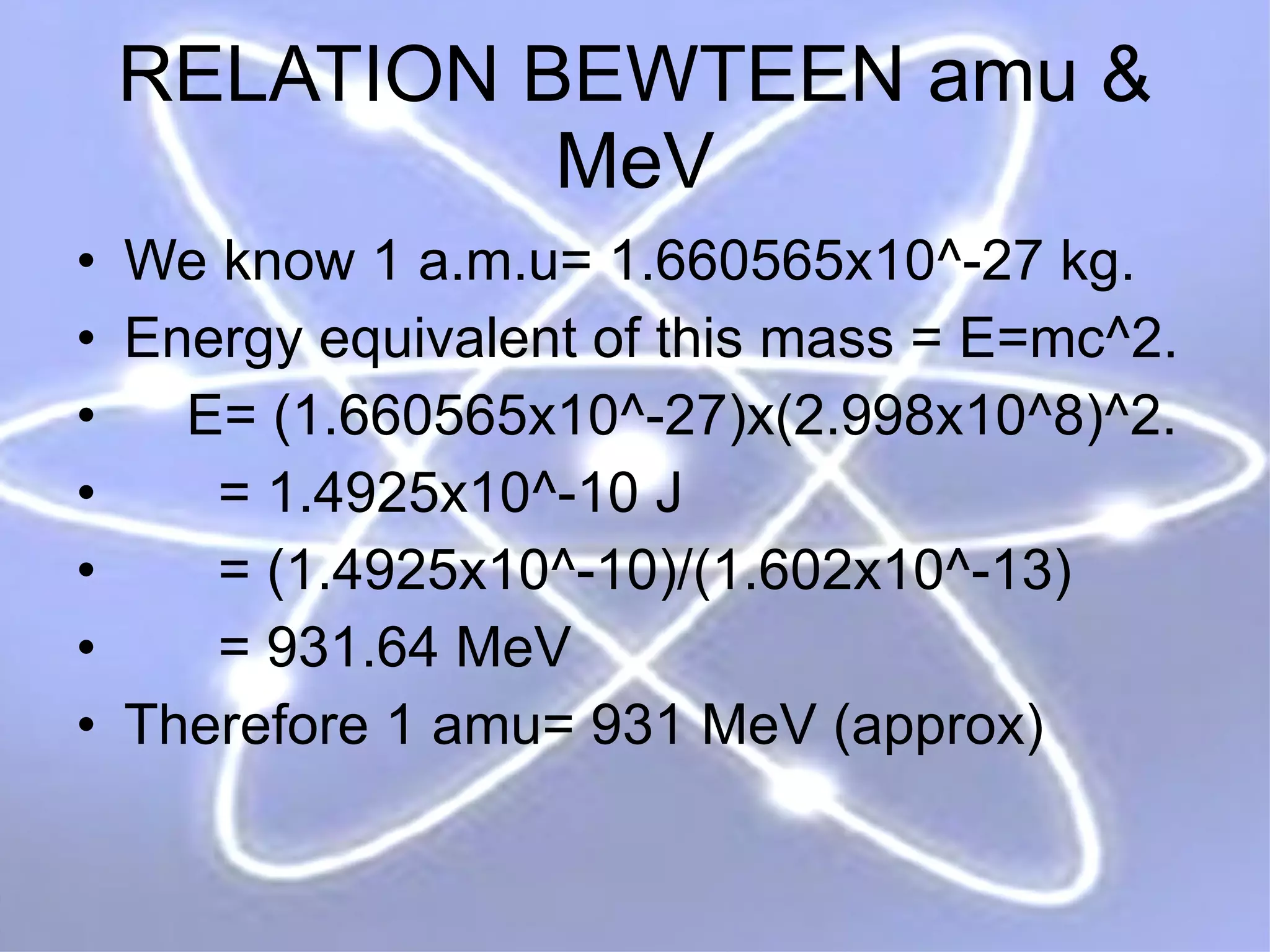
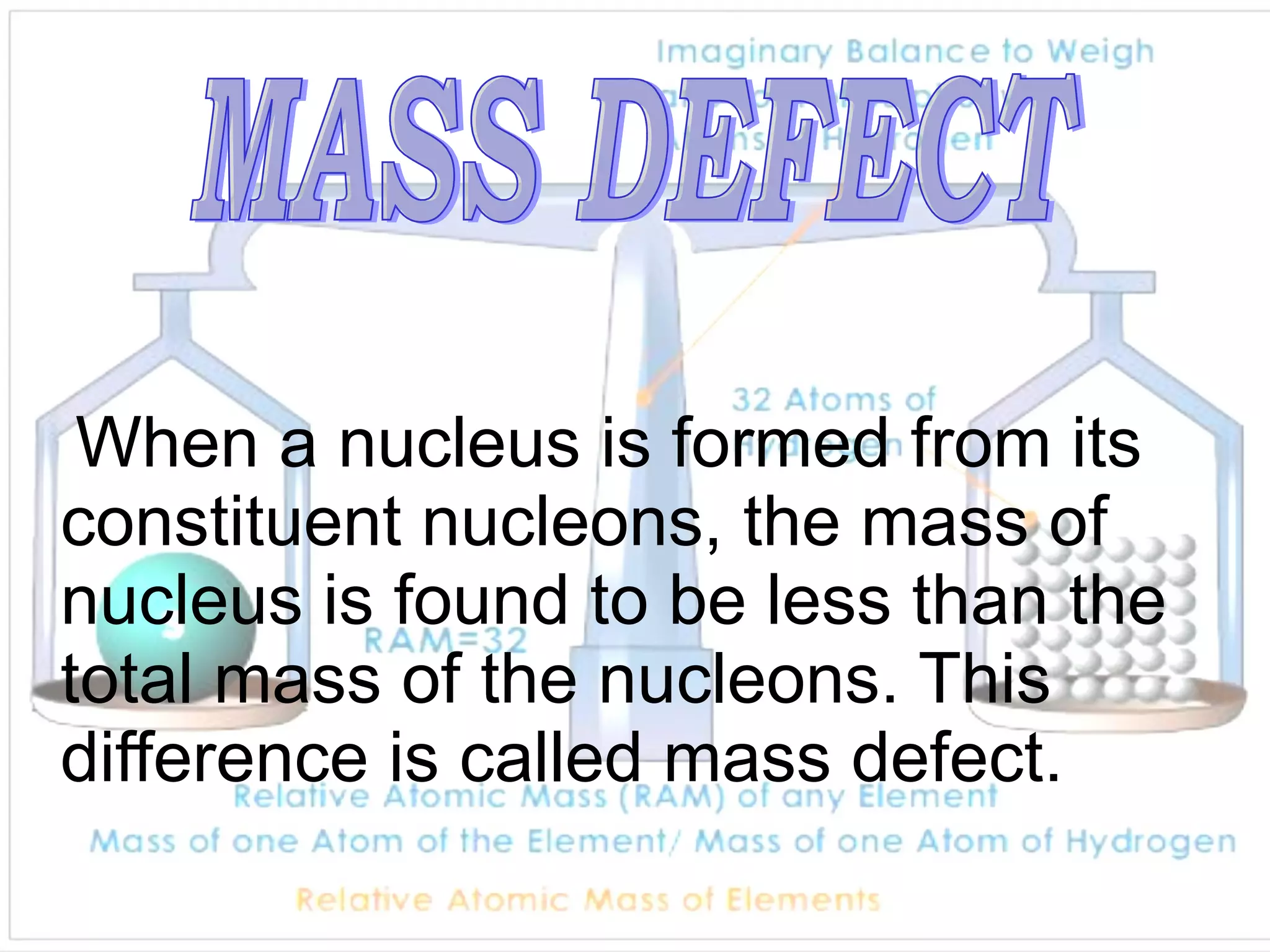
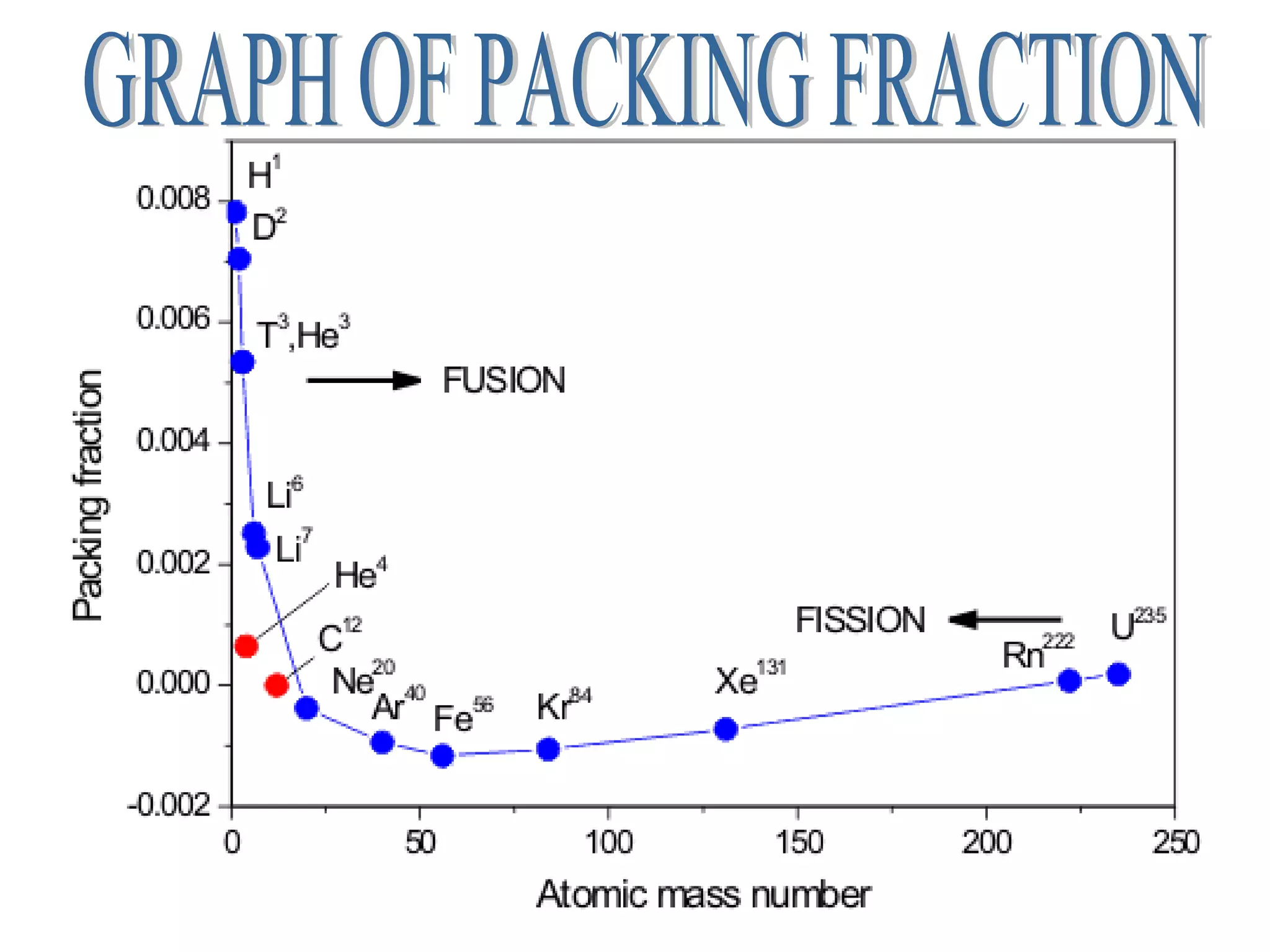
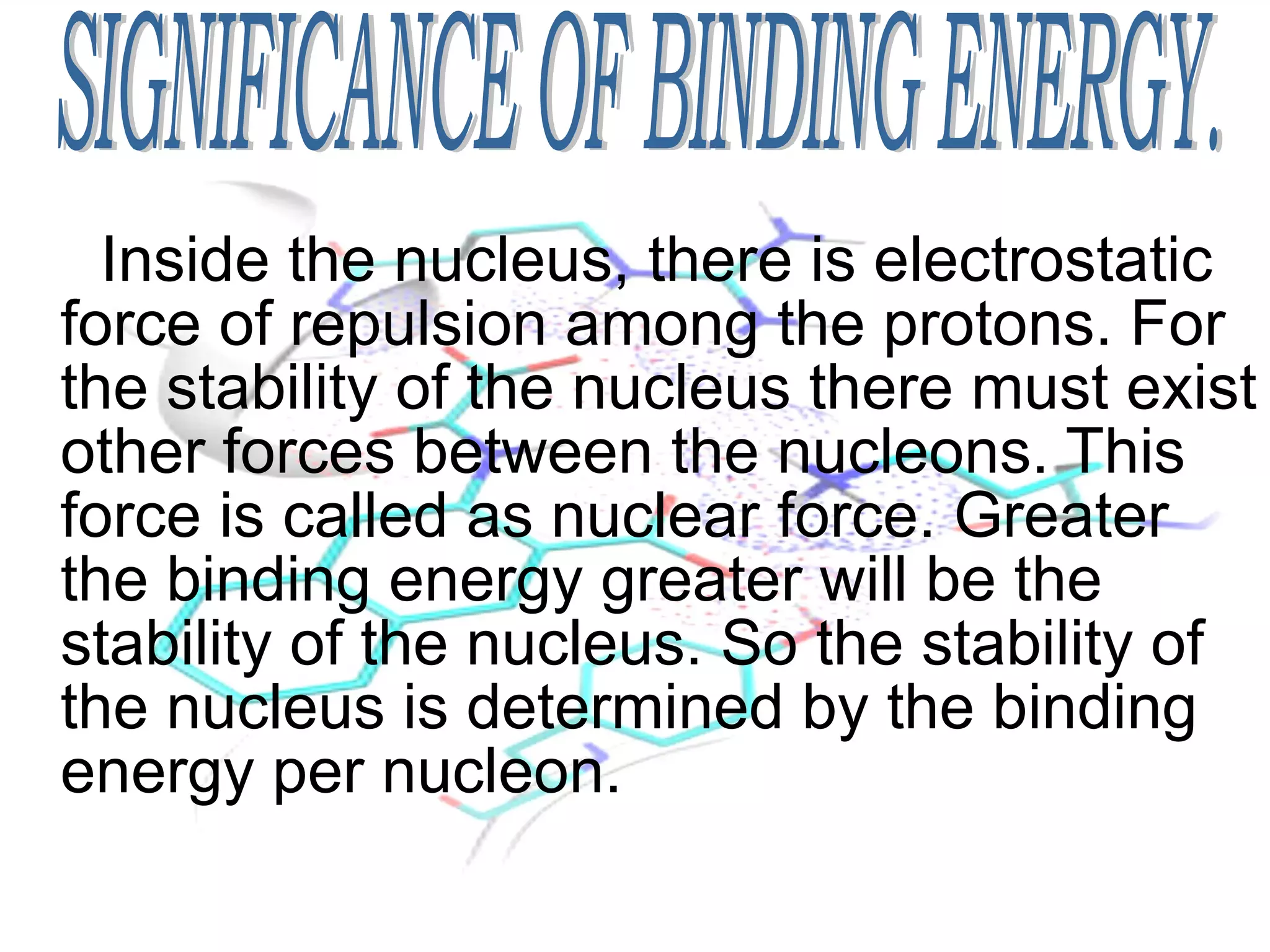
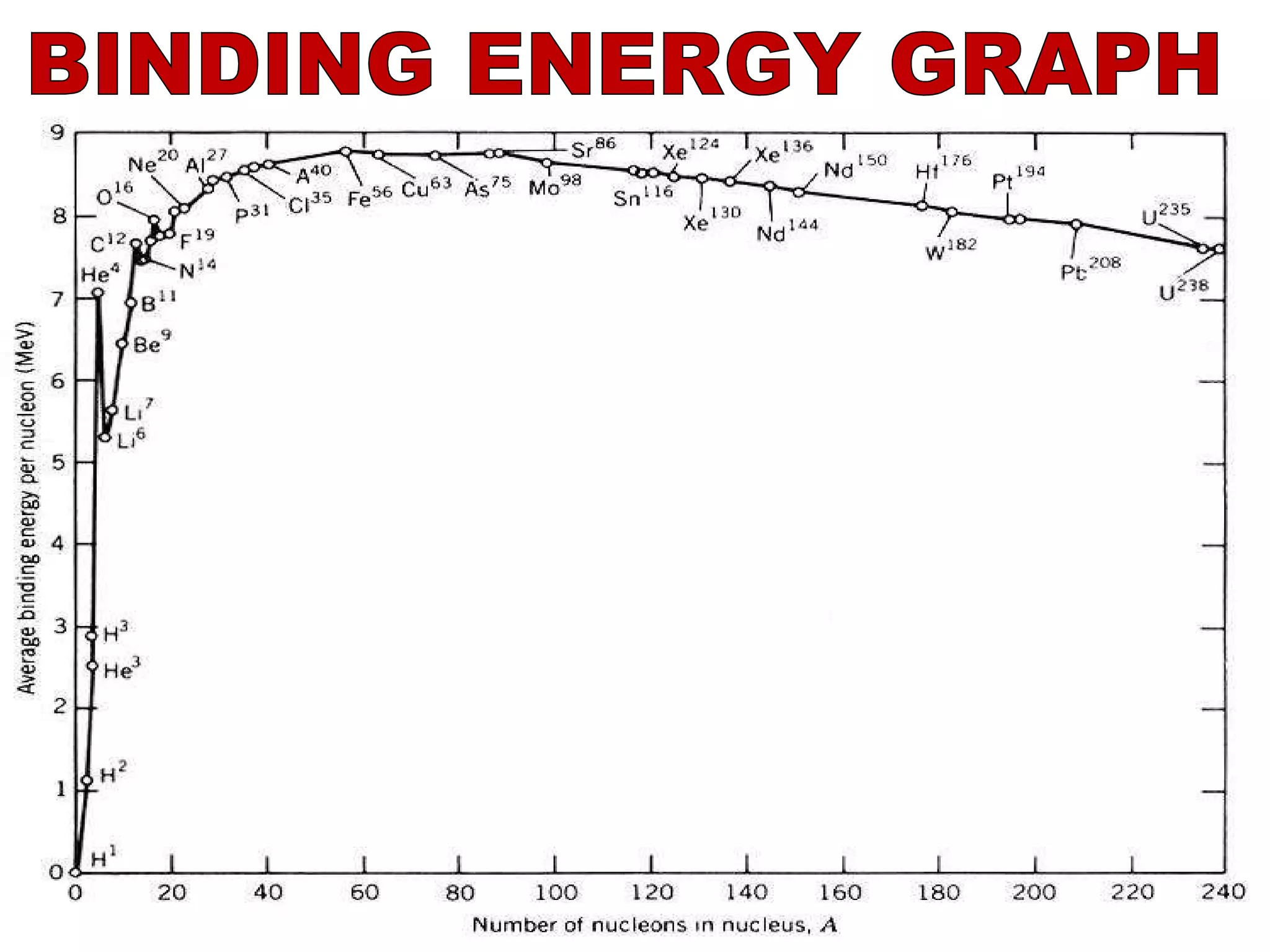
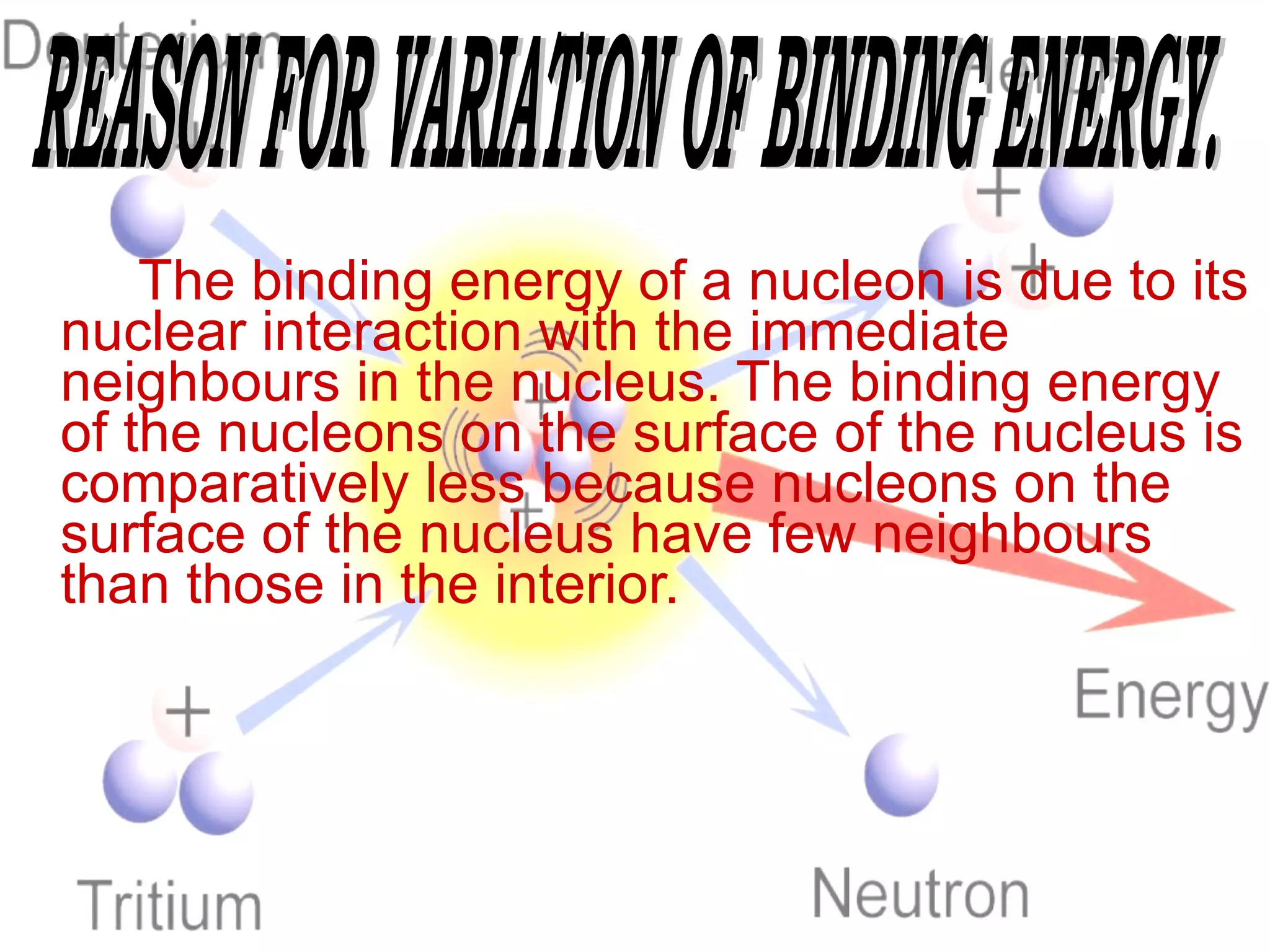
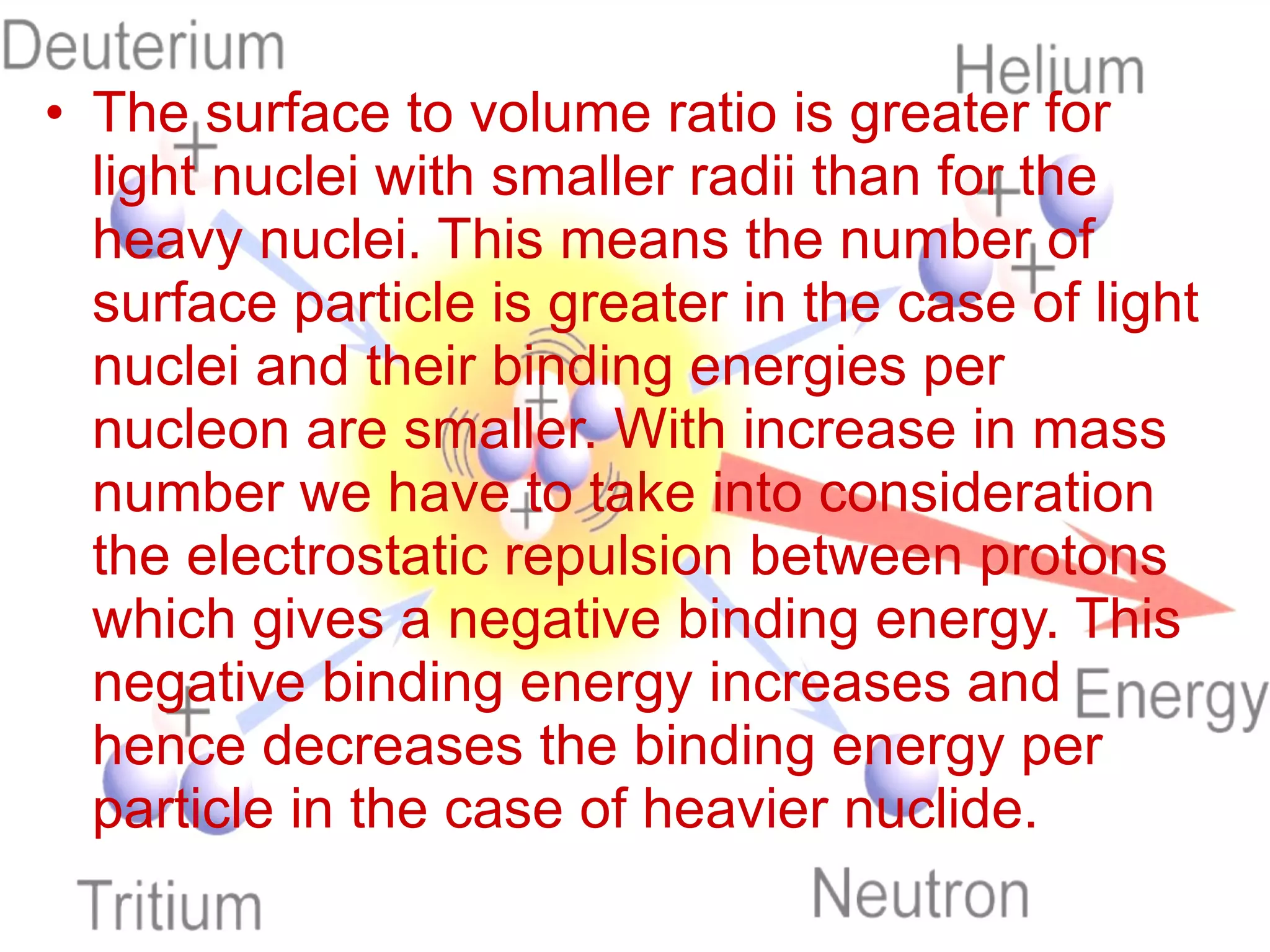
1. Lord Rutherford discovered the nucleus through alpha particle scattering experiments, finding that atoms consist of a small, dense, positively charged nucleus surrounded by orbiting electrons.
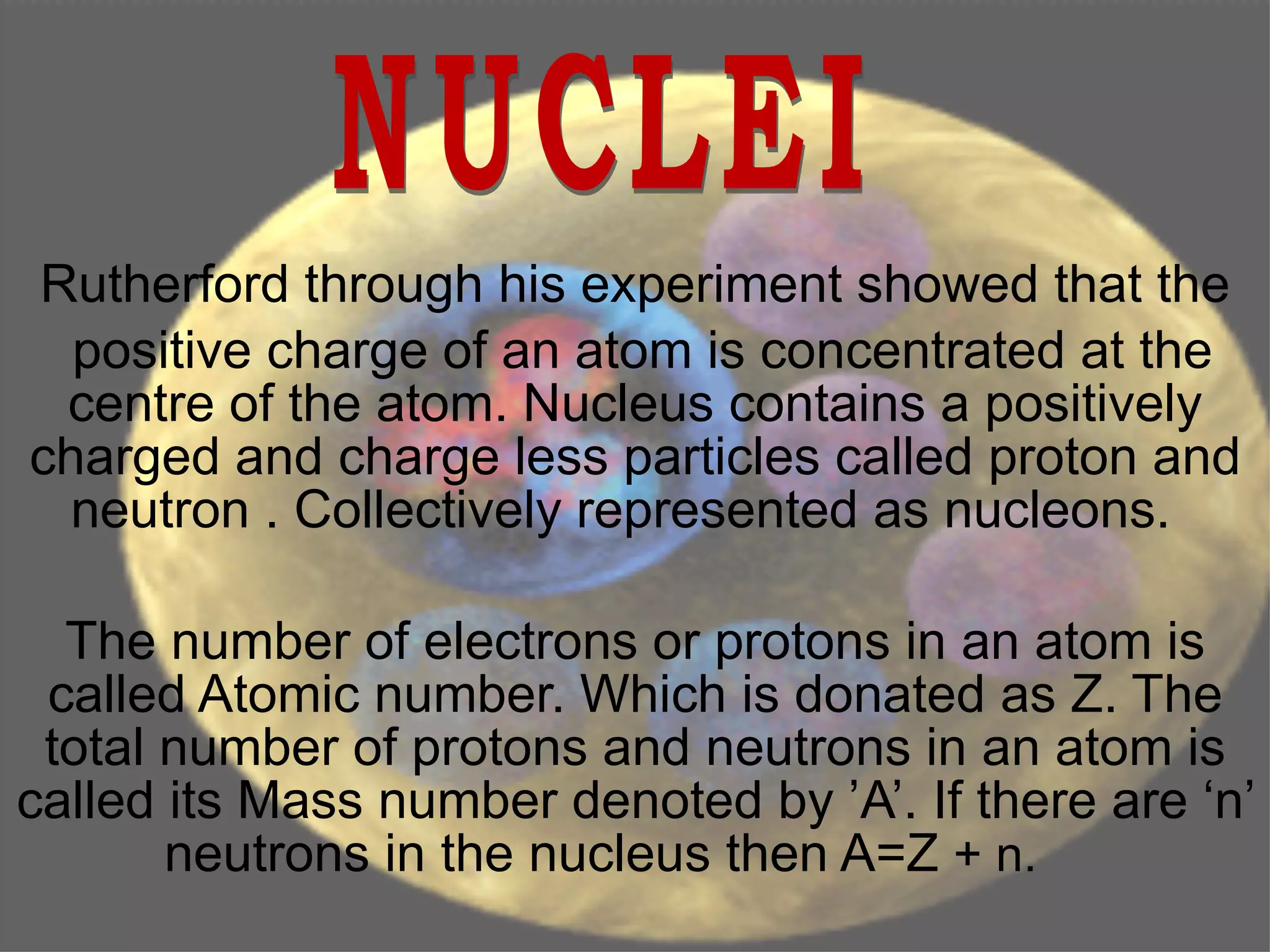
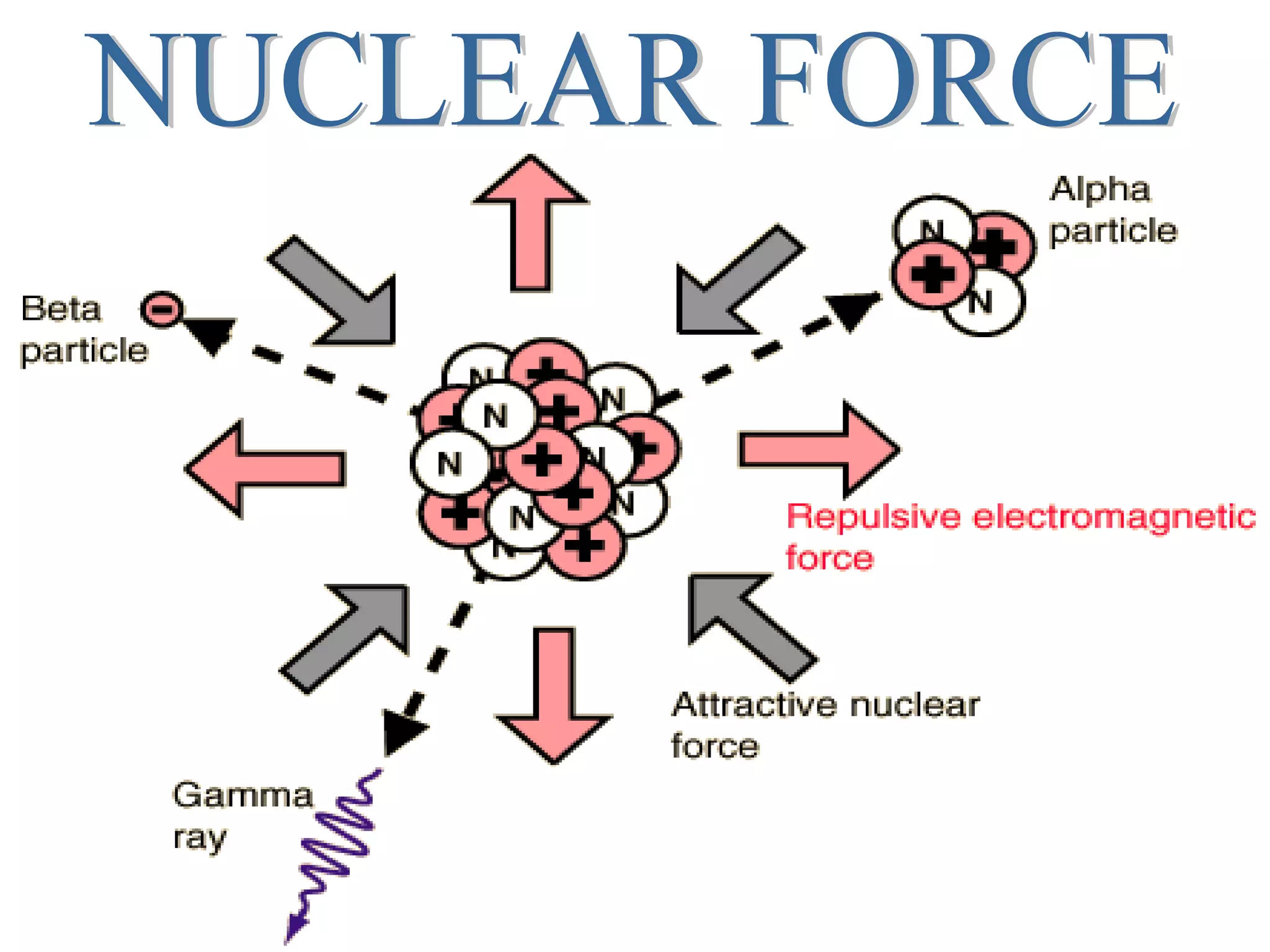
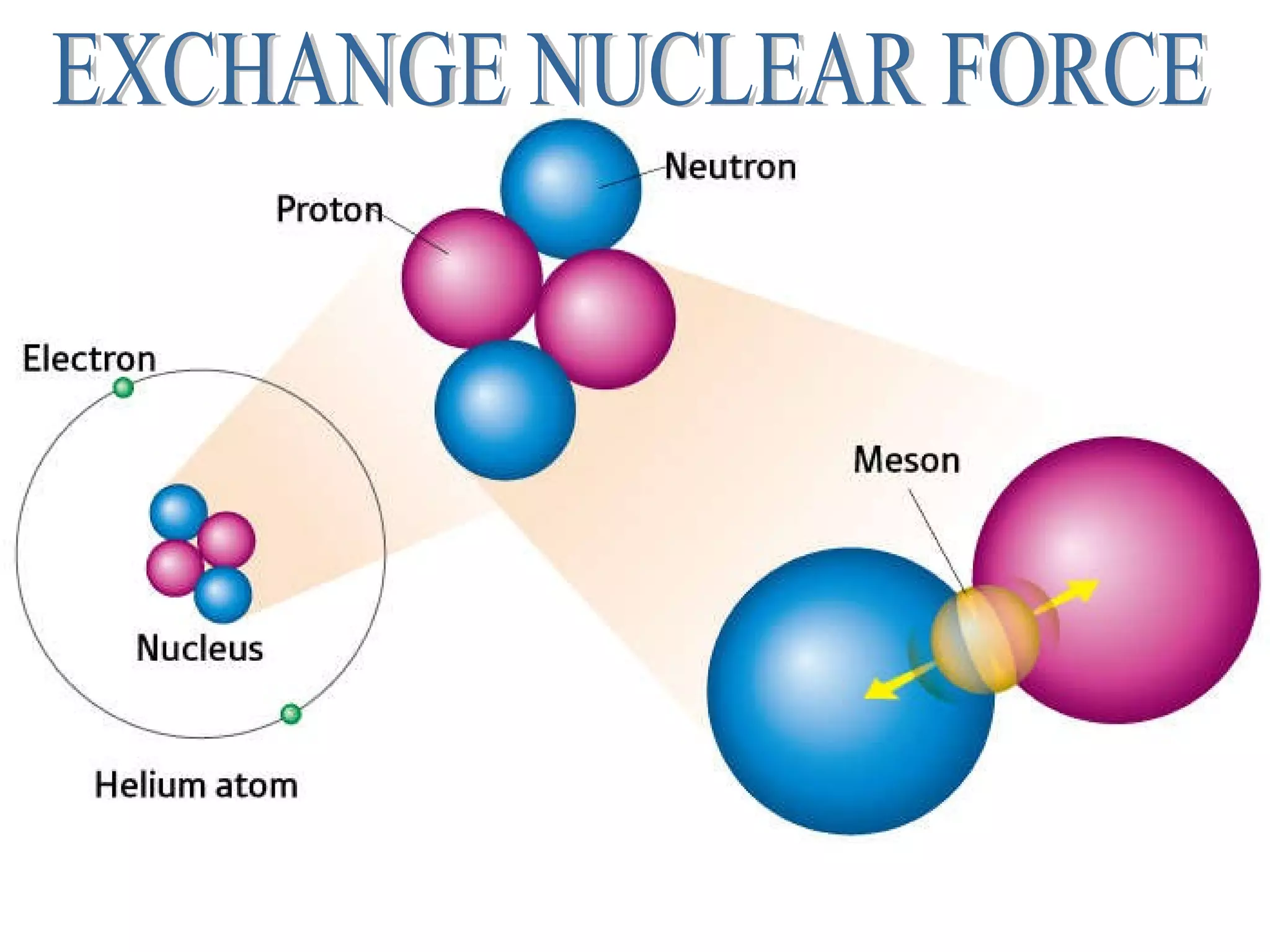
2. The nucleus contains positively charged protons and neutral neutrons, collectively called nucleons. The number of protons is the atomic number and the total number of protons and neutrons is the mass number.
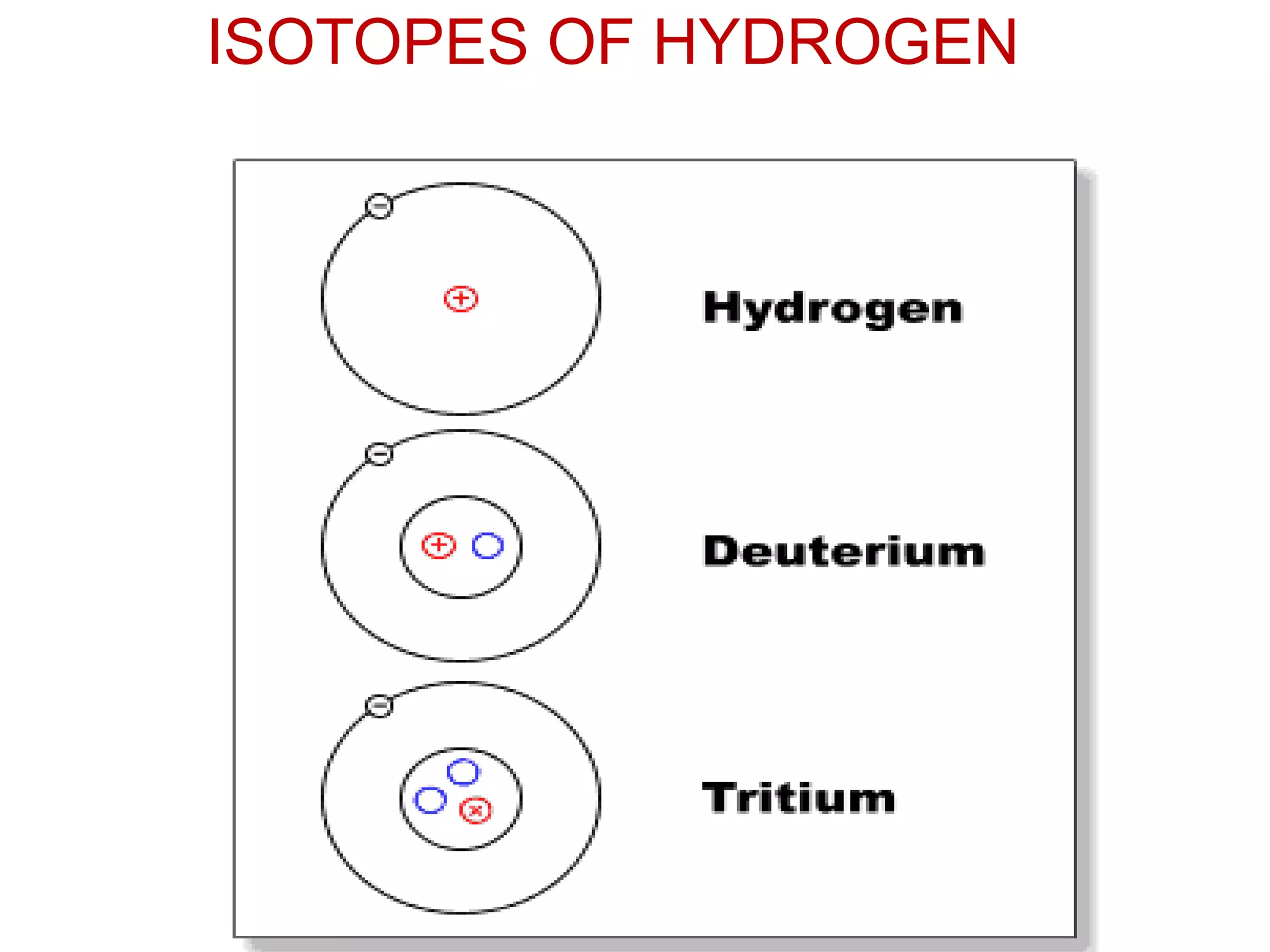
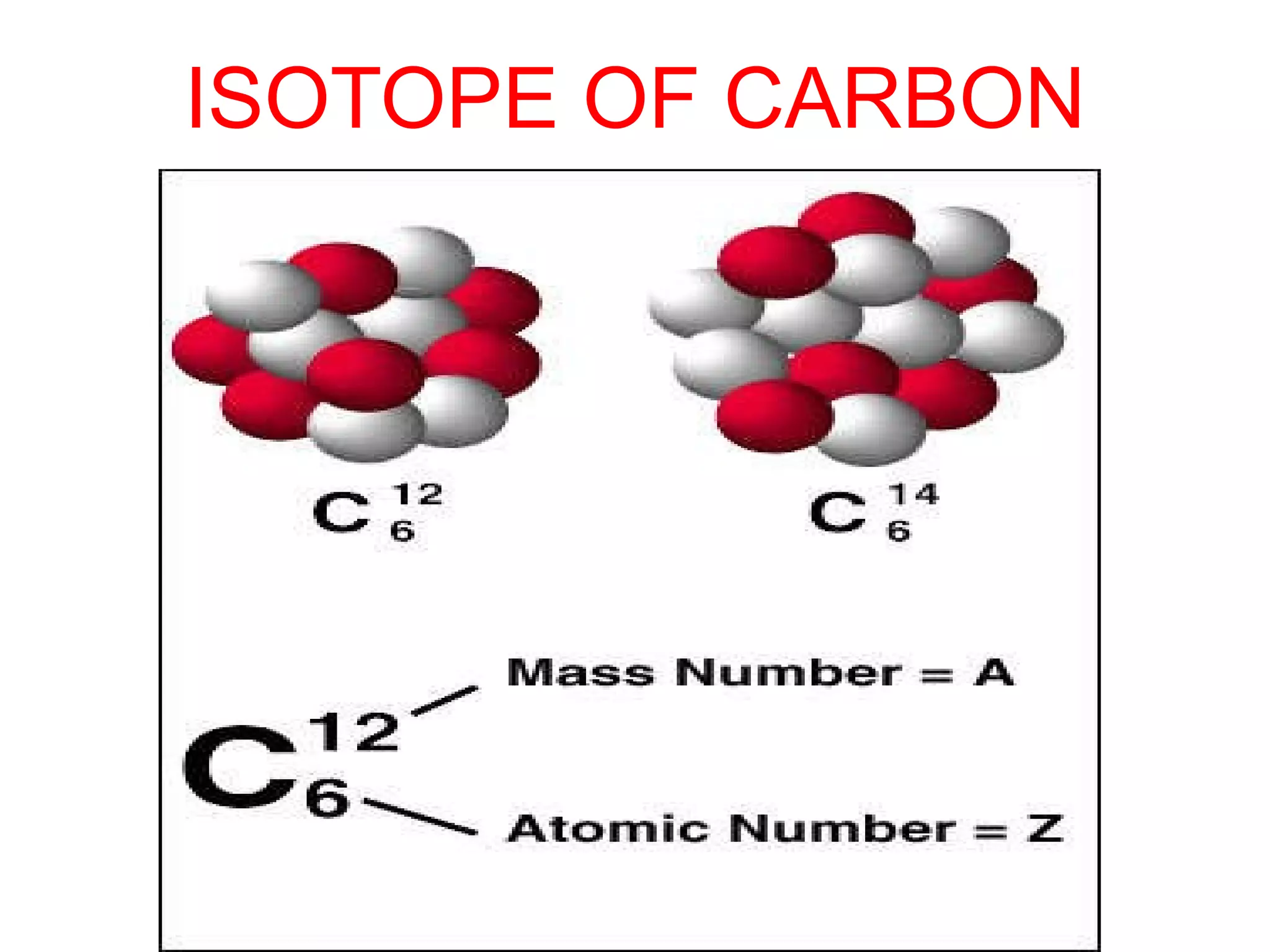
3. Isotopes are atoms with the same atomic number but different mass numbers, such as the three isotopes of hydrogen: deuterium, ordinary hydrogen, and tritium.