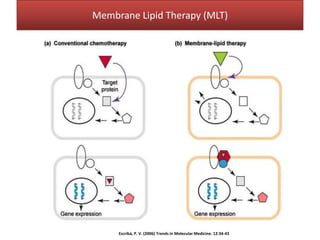
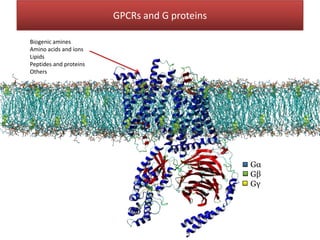
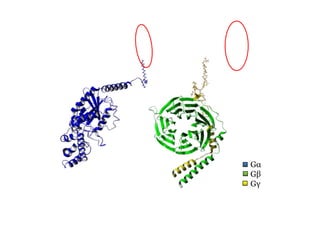
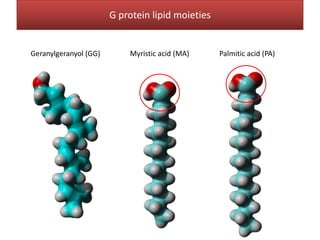
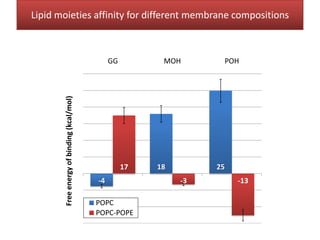
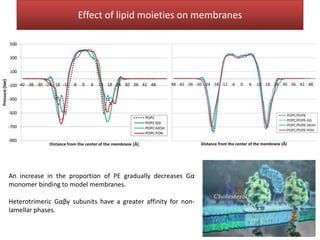
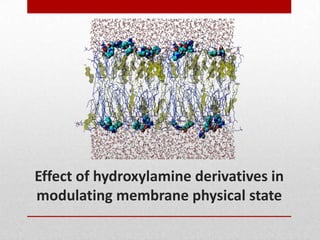
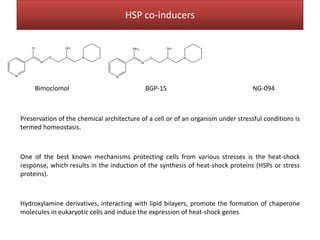
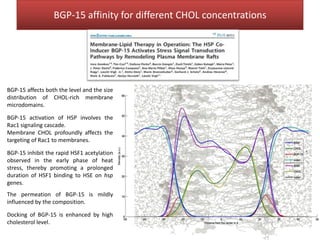
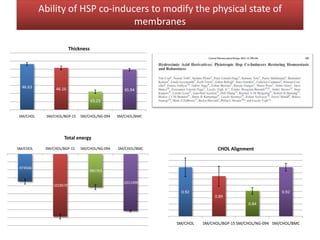
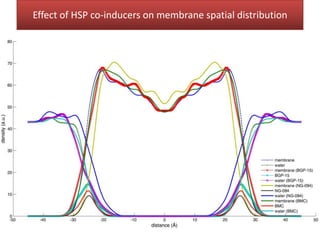
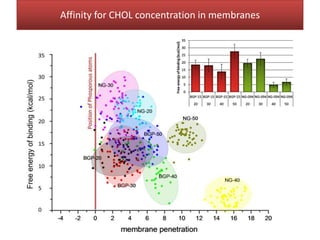
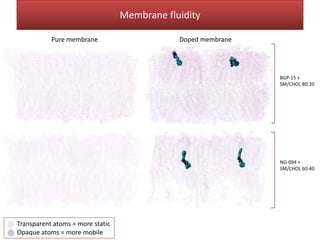
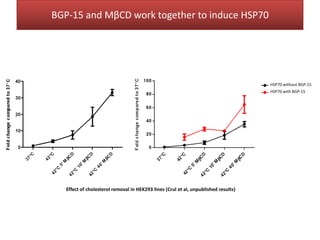
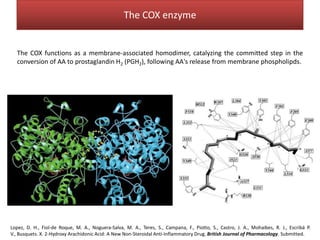
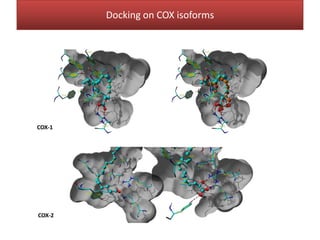
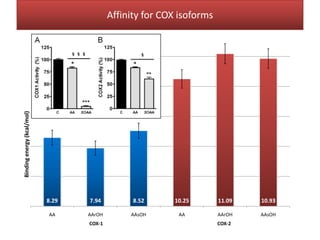
This document summarizes Federica Campana's doctoral thesis on investigating drug-cell membrane interactions using molecular dynamics simulations. The thesis examines how membrane composition influences the effects of membrane fluidizers and heat shock protein co-inducers. It also analyzes the binding of anti-inflammatory molecules like hydroxyarachidonic acid to cyclooxygenase enzymes. The overall goal is to better understand how drug molecules interact with and modulate lipid bilayer properties at a molecular level.