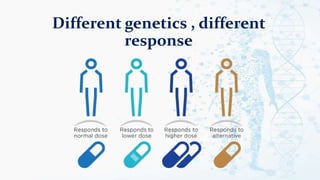
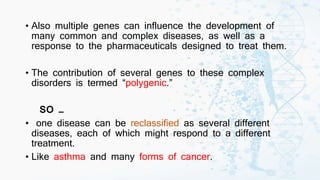
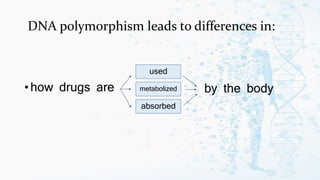
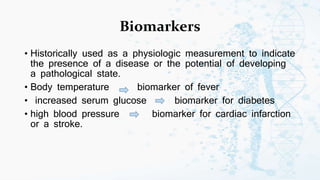
The document discusses personalized medicine, which tailors medical treatments to individual patient characteristics, highlighting the importance of pharmacogenetics in determining drug efficacy based on genetic variations. It outlines the historical context, benefits of personalized approaches, and the potential for better treatment outcomes while addressing challenges related to insurance and patient privacy. Future prospects aim for improved drug success rates and a deeper understanding of complex diseases influenced by genetics and environmental factors.