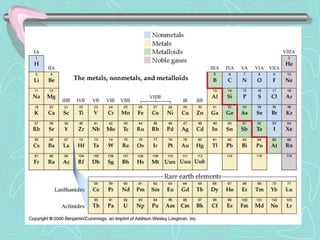
The document summarizes key information about the periodic table, including that there are 118 known elements as of 2010, with 94 found naturally on Earth. It describes how chemistry was disorganized before the periodic table was developed by Dmitri Mendeleev, who arranged elements in rows by atomic weight and columns by chemical properties, leaving spaces for undiscovered elements. The current periodic table arranges elements by atomic number in rows called periods and columns called groups, which have similar properties based on their valence electrons. Key groups described include alkali metals, alkaline earth metals, transition metals, and noble gases.