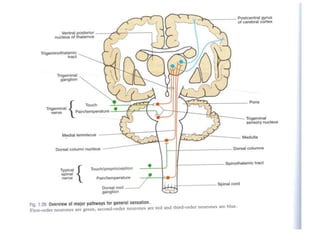
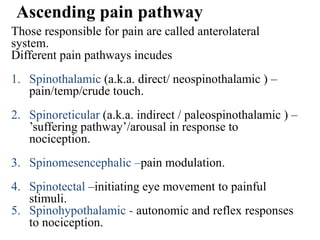
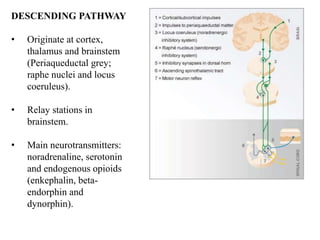
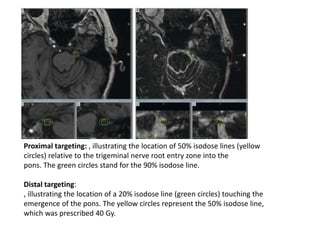
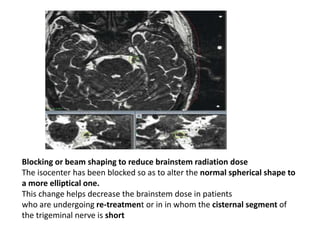
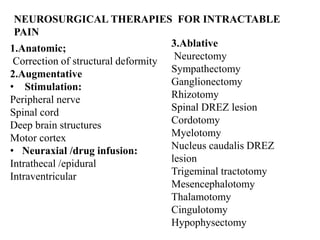
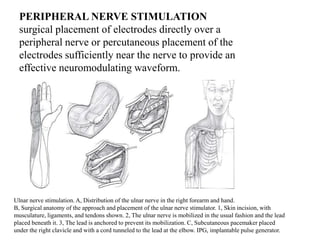
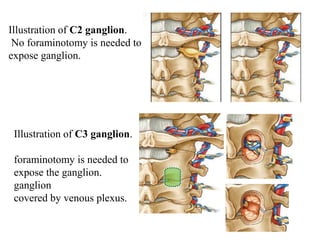
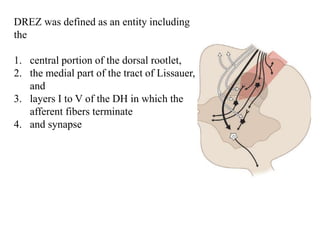
This document discusses pain pathways and treatments for pain. It begins by defining pain according to the IASP and describing the sensory and affective dimensions of pain. It then outlines the ascending pain pathway from peripheral receptors to the cortex. Several descending pain pathways are also described originating from the cortex, thalamus, and brainstem. Common craniofacial pain syndromes and their possible pathways are listed. Surgical and non-surgical treatment options for intractable pain are summarized, including neurostimulation, ablative procedures, and neuromodulation therapies.