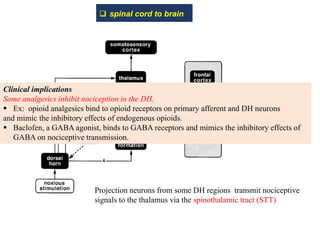
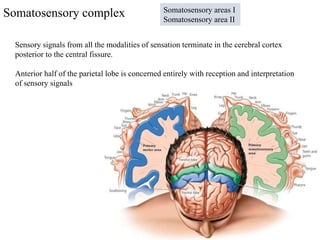
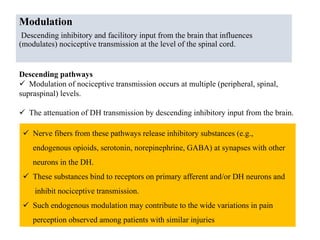
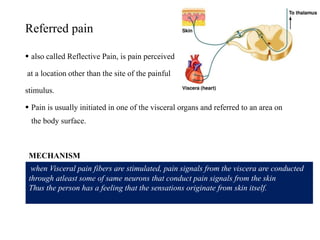
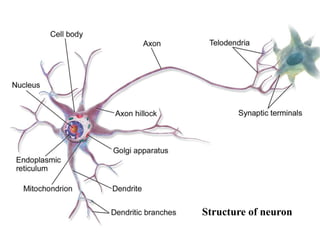
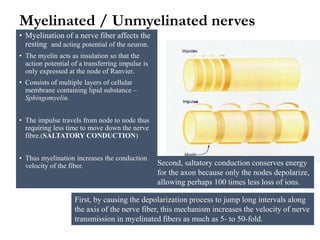
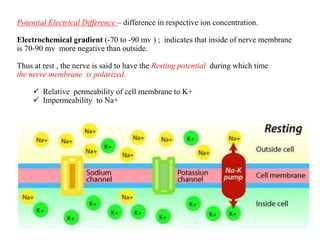
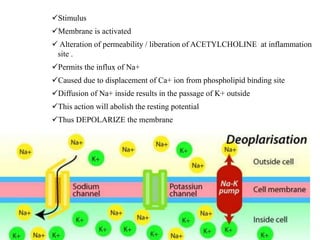
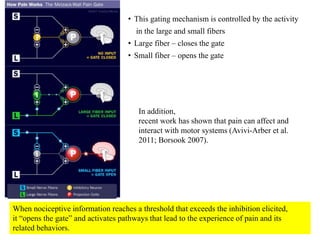
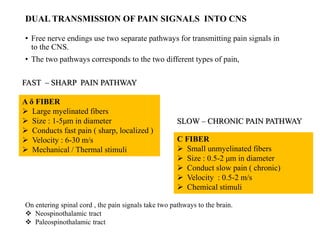
This document summarizes information about pain and taste pathways. It discusses definitions of pain, types of pain, factors affecting pain perception, nerve structure and conduction, sensory receptors, pain theories, and neural pathways. It also covers taste receptors, taste transmission to the central nervous system, taste disorders, and their clinical evaluation and management.

















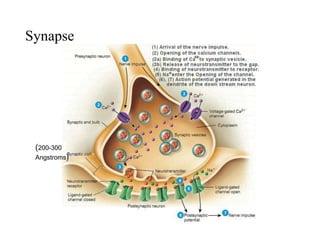
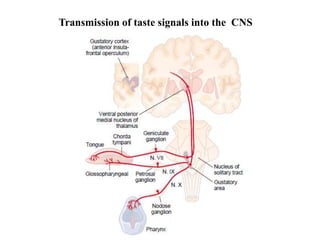
![Transmission
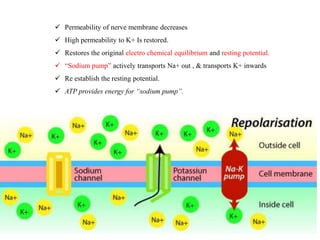
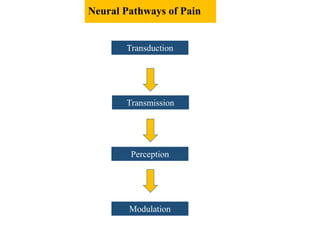
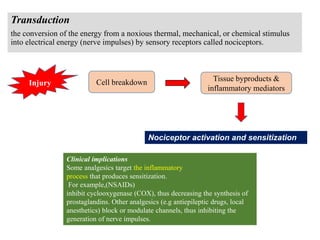
the transmission of these neural signals from the site of transduction (periphery) to
the spinal cord and brain.
Periphery to spinal cord
sensory nerve impulses travel via axons dorsal horn
propagate nerve impulses
release of excitatory amino acids (EAAs)
at synapse
Activated DH projection neurons
forward nociceptive impulses toward the brain
Spinal interneurons
release inhibitory amino acids (e.g.,
$-aminobutyric acid [GABA] &
neuropeptides (endogenous opioids)
bind to receptors on primary afferent and DH
neurons
inhibit nociceptive transmission](https://image.slidesharecdn.com/painseminar-190228153141/85/pain-and-taste-pathway-34-320.jpg)