Embed presentation
Download to read offline




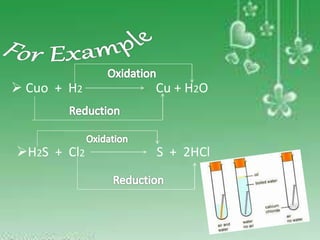


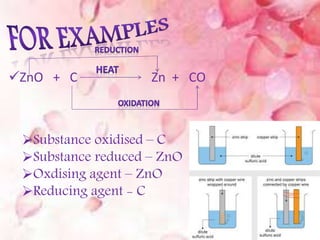
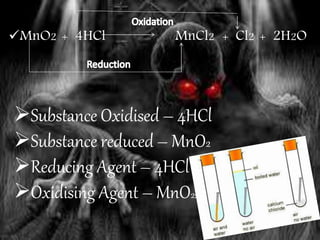

Oxidation is the addition of oxygen to a substance or removal of hydrogen, while reduction is the addition of hydrogen or removal of oxygen. An oxidizing agent provides oxygen for oxidation reactions and removes hydrogen, while a reducing agent provides hydrogen for reduction reactions and removes oxygen. Examples of oxidation-reduction reactions are provided along with identification of the oxidized substance, reduced substance, oxidizing agent, and reducing agent in each reaction.









