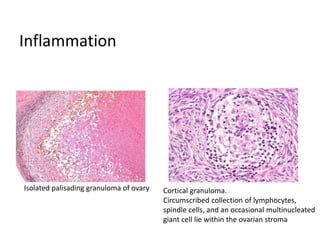
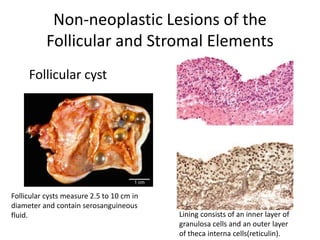
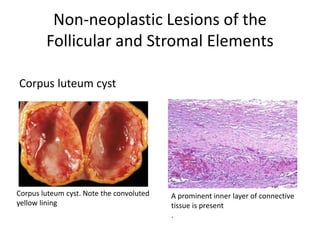
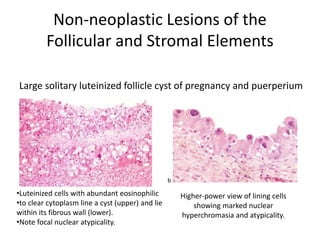
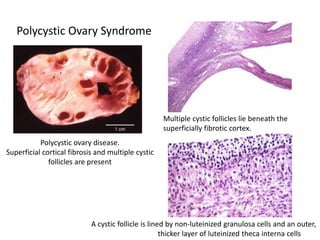
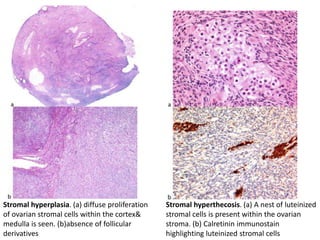
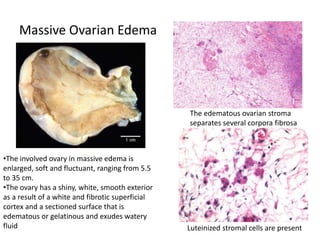
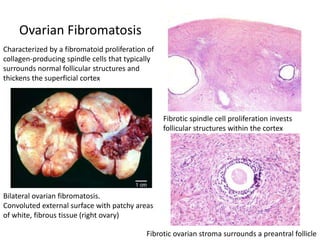
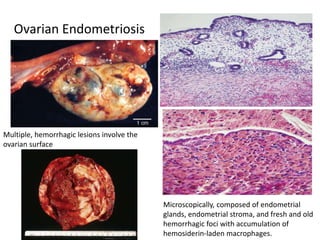
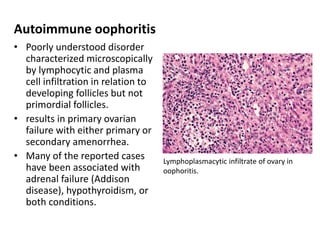
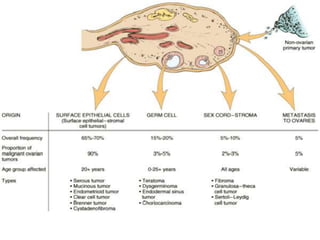
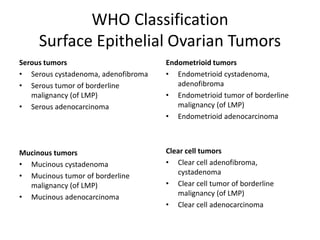
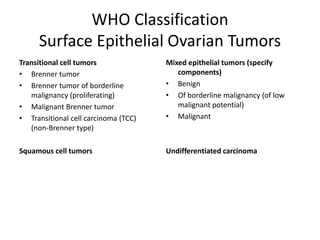
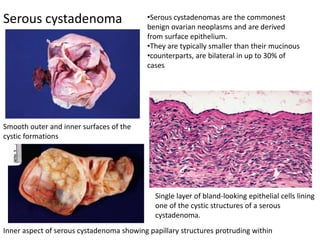
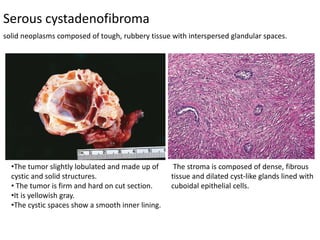
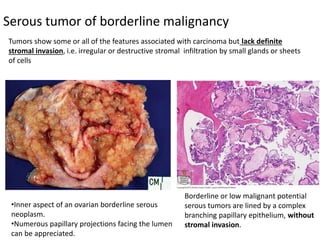
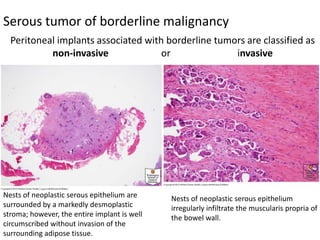
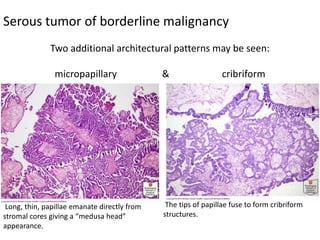
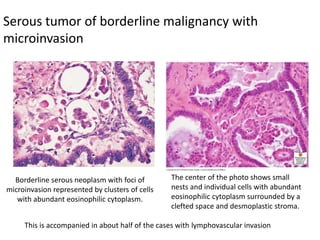
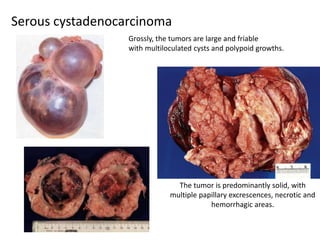
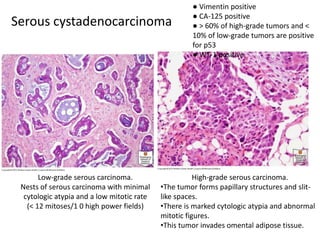
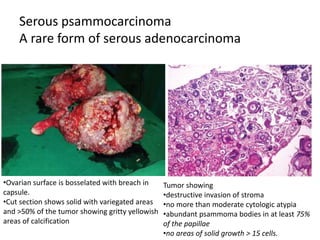
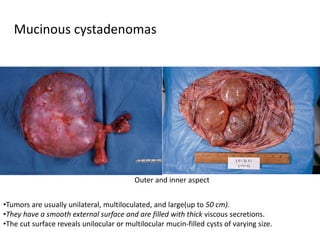
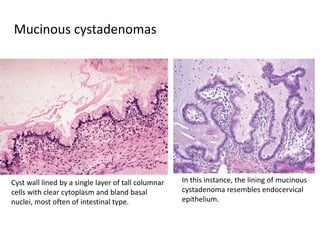
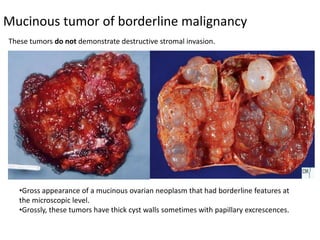
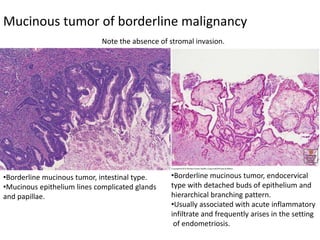
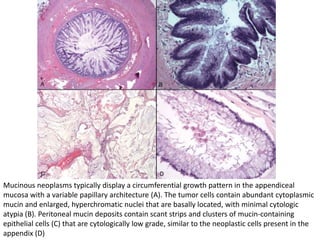
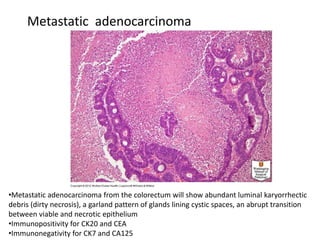
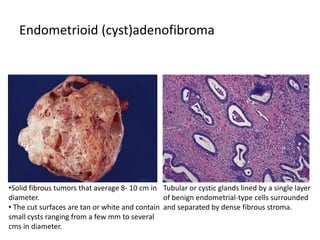
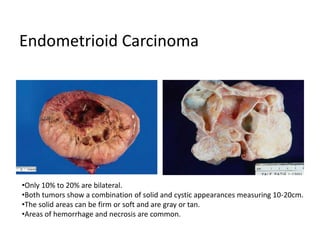
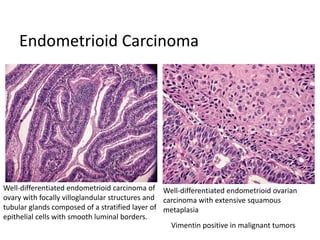
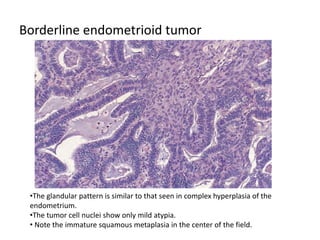
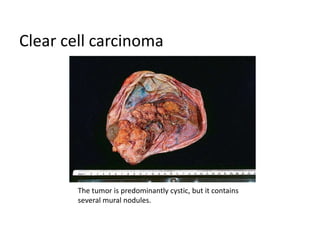
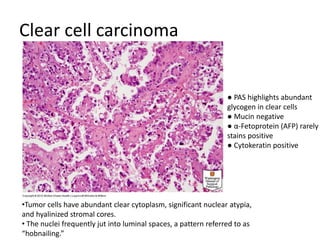
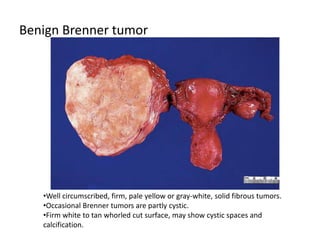
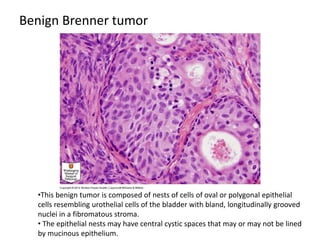
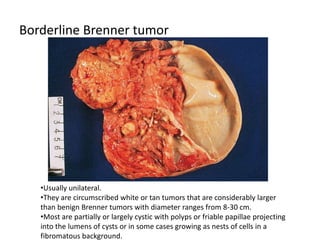
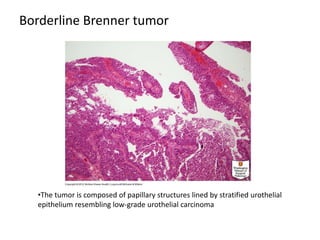
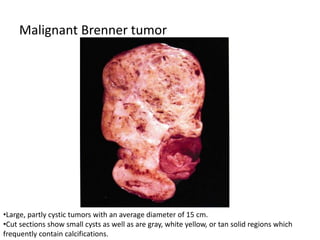
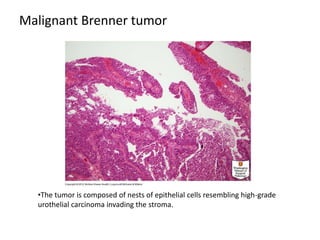
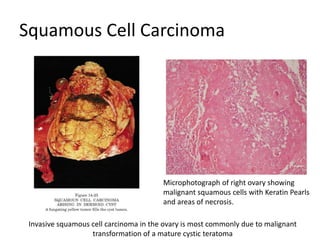
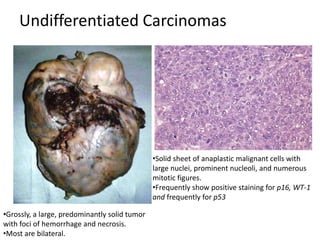
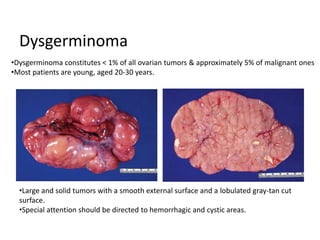
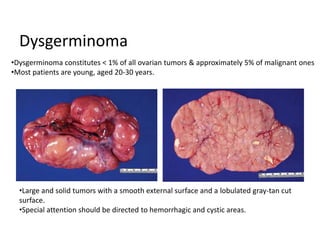
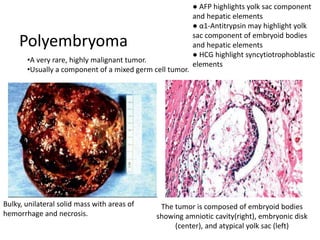
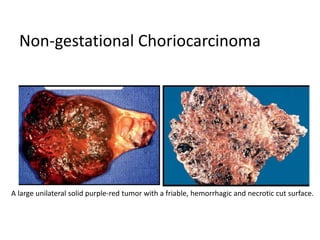
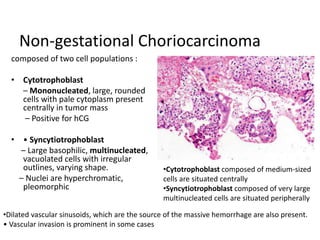
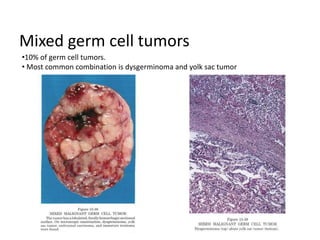
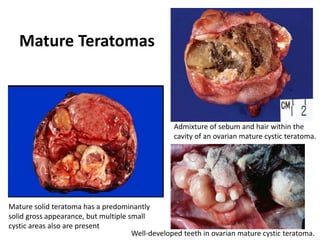
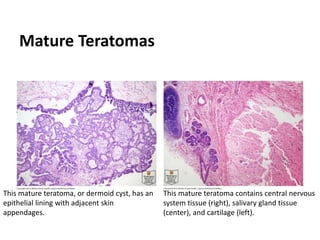
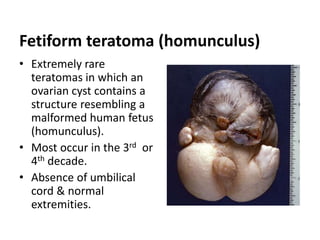
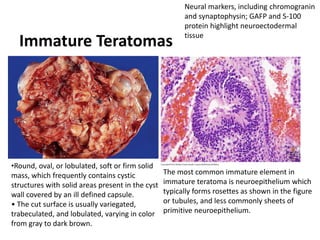
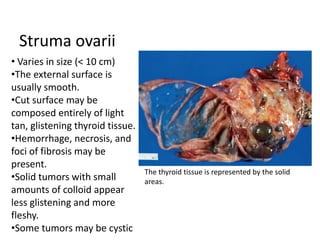
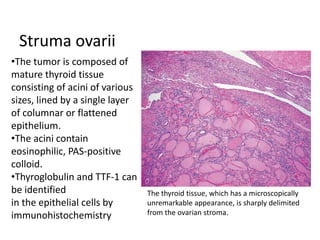
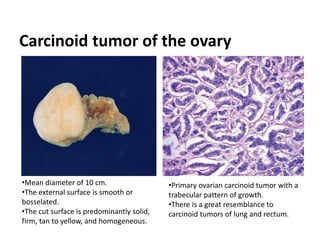
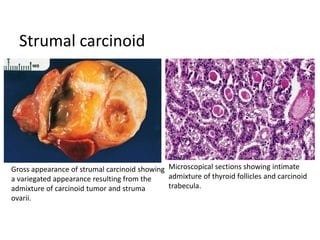
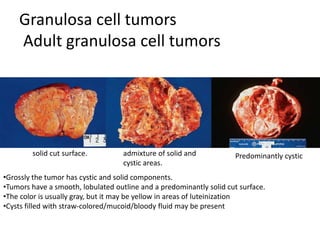
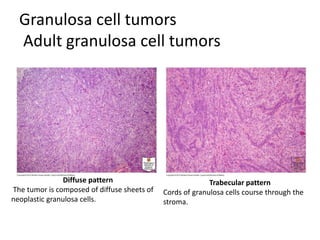
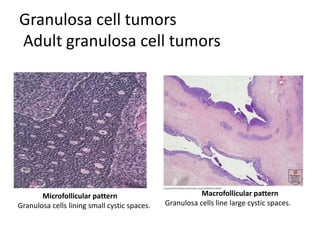
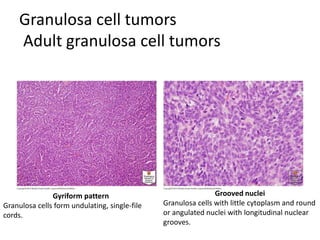
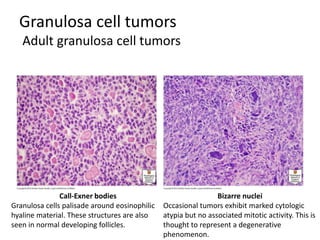
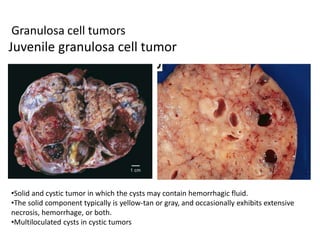
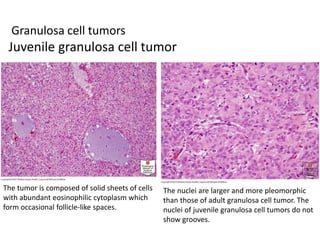
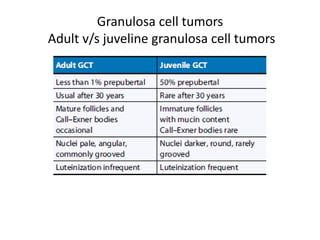
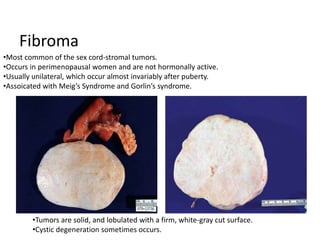
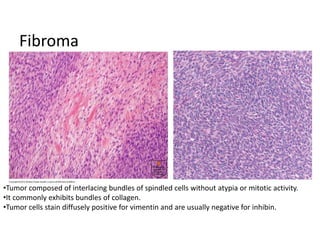
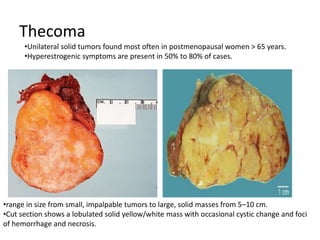
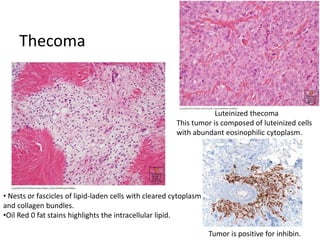
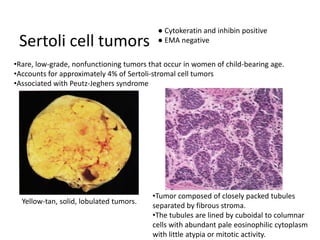
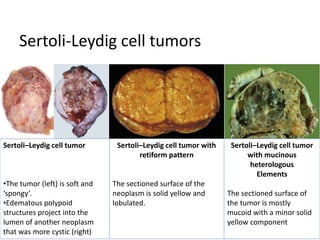
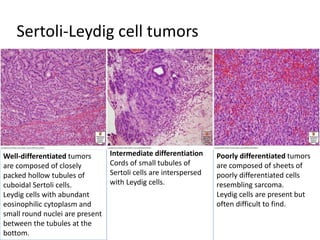
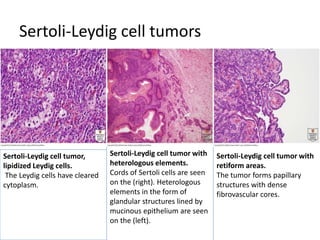
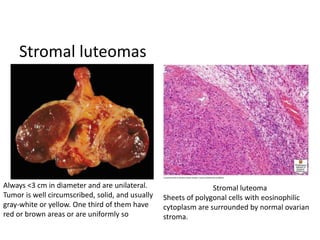
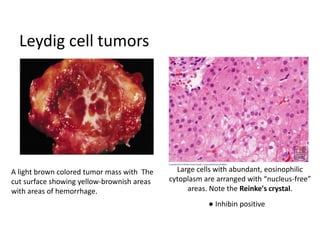
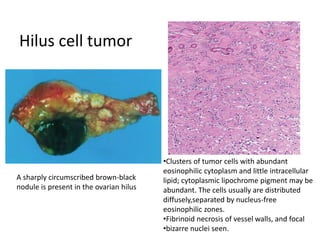
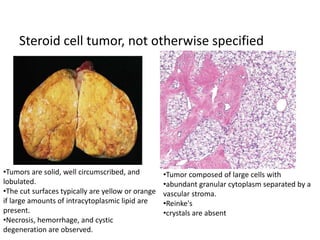
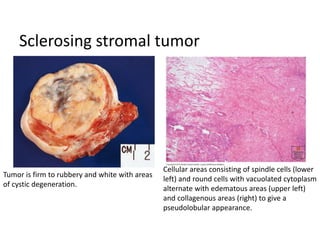
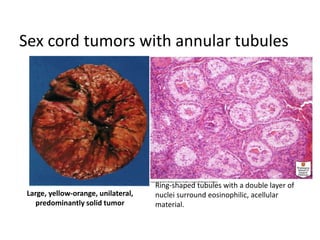
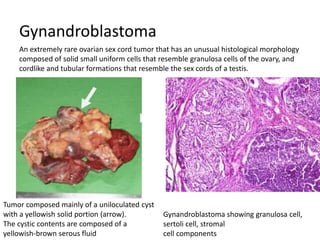
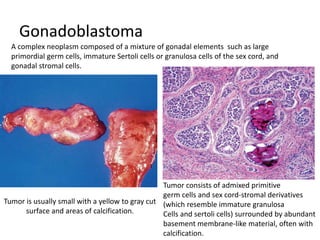
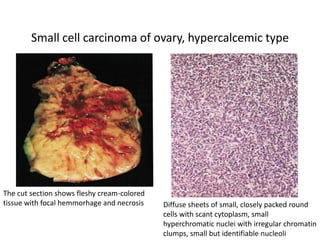
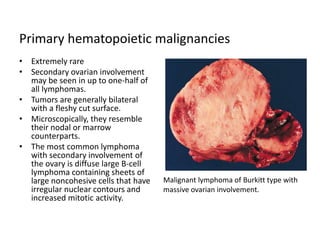
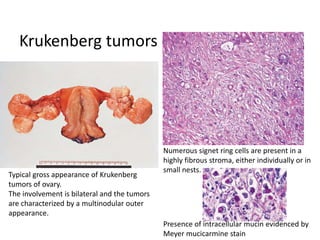
This document provides an overview of ovarian lesions, both non-neoplastic and neoplastic. It discusses various congenital lesions, infections, inflammatory conditions, and functional cysts that can involve the ovaries in a non-neoplastic manner. It then covers the WHO classification of ovarian tumors and provides detailed descriptions and microscopic images of the various subtypes of epithelial ovarian cancer, including serous, mucinous, endometrioid, clear cell carcinomas and others. Rare variants like serous psammocarcinoma are also described.