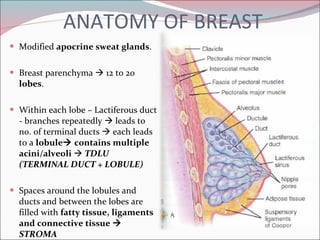
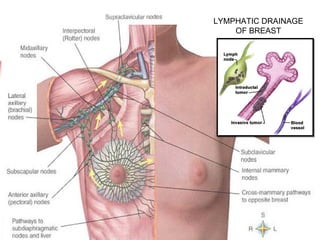
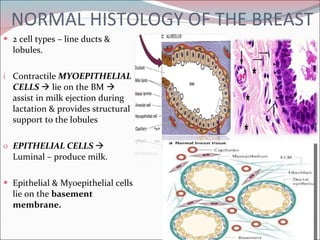
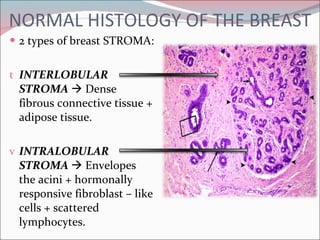
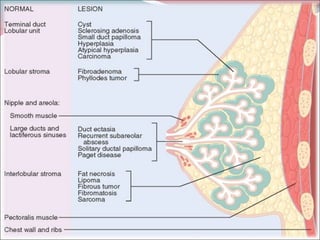
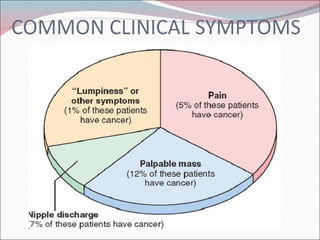
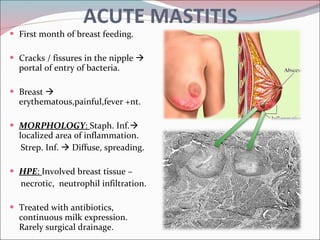
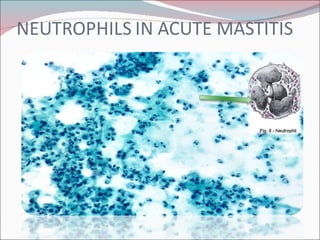
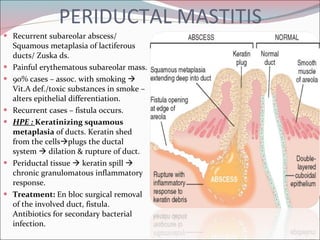
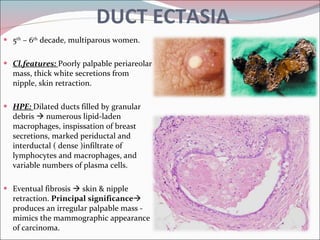
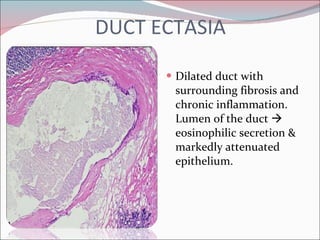
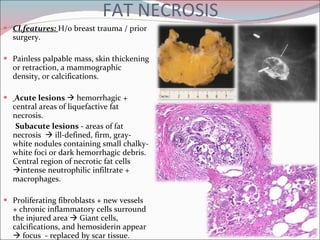
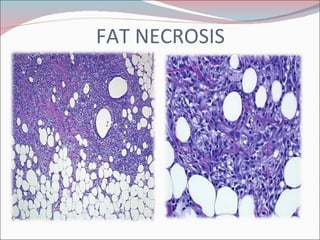
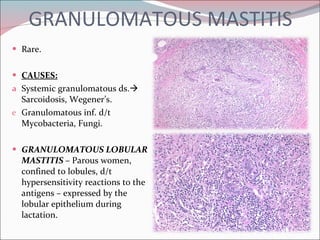
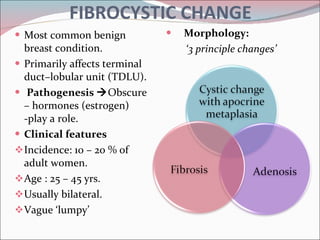
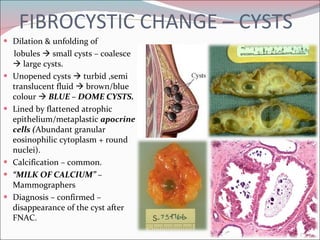
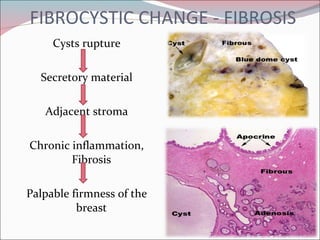
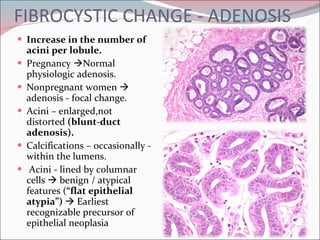
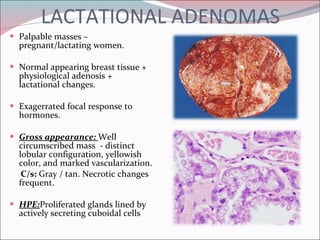
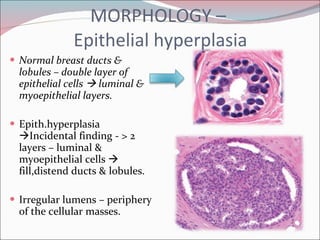
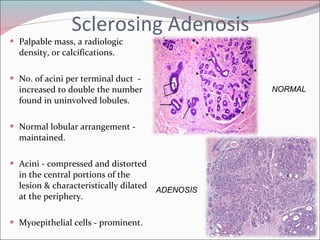
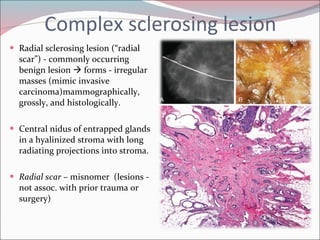
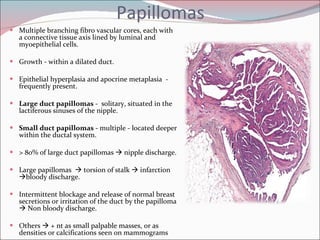
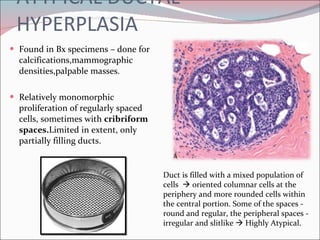
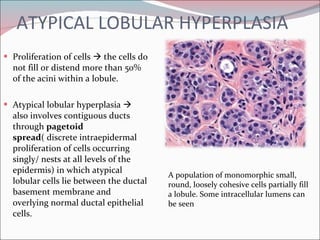
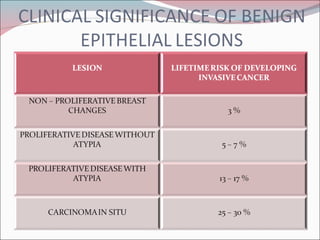
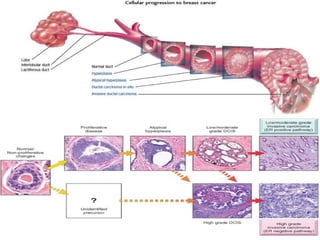
The document summarizes the anatomy and histology of the normal breast as well as various benign breast conditions. It describes the lobes, ducts, lobules and stroma of the breast. It then discusses various benign breast diseases and alterations including acute mastitis, periductal mastitis, duct ectasia, fat necrosis, granulomatous mastitis and various proliferative breast diseases without atypia.