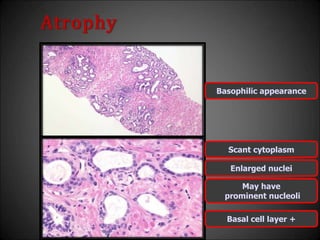
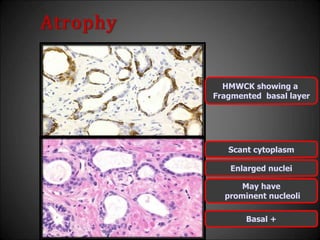
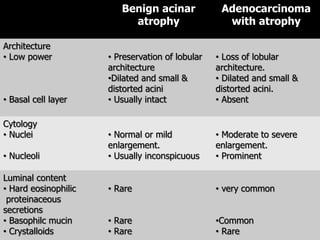
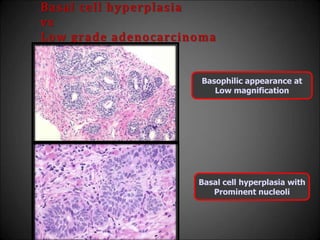
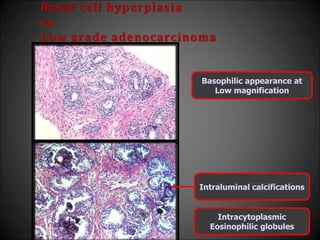
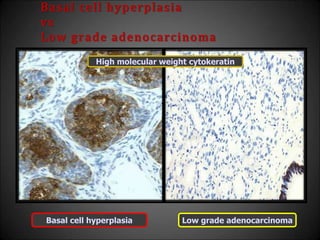
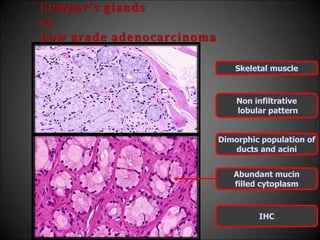
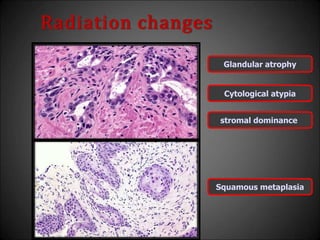
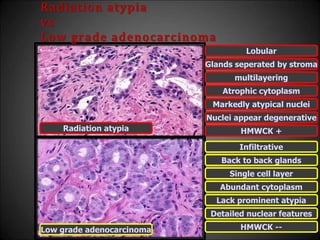
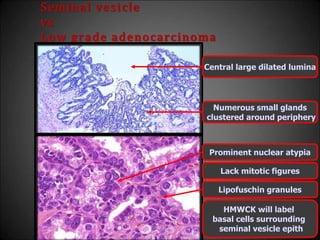
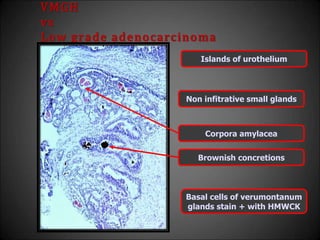
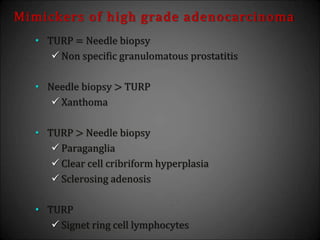
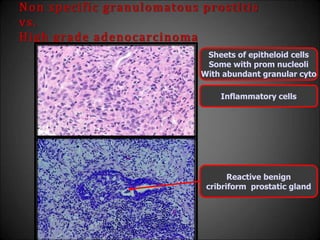
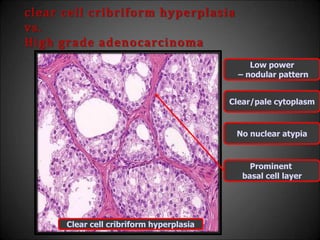
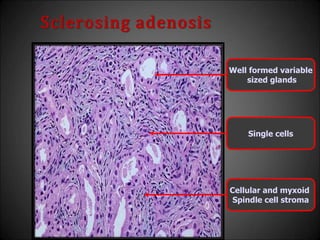
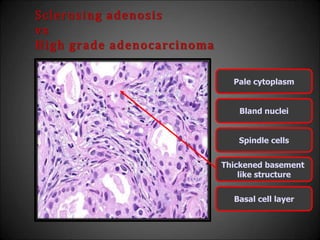
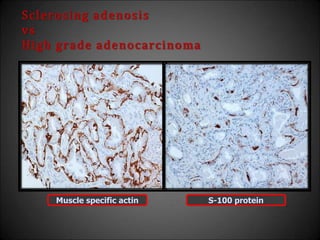
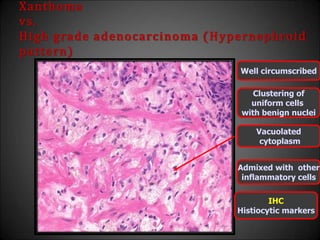
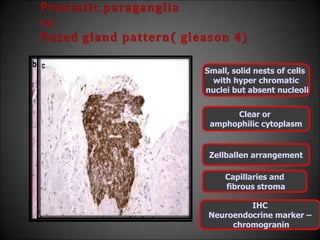
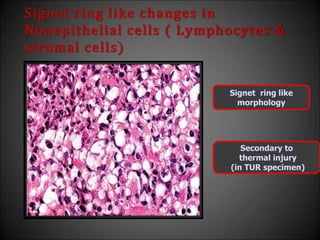
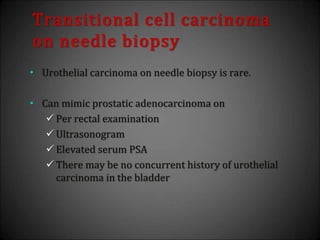
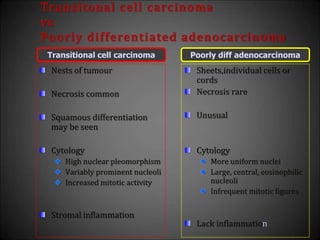
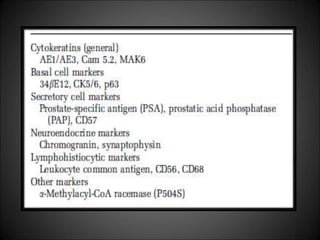
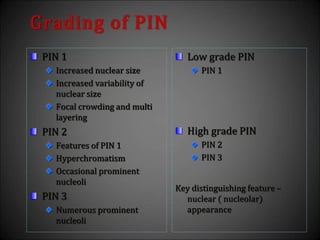
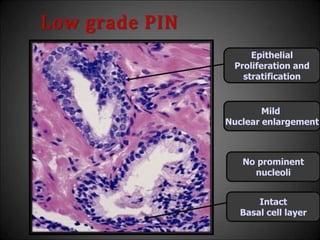
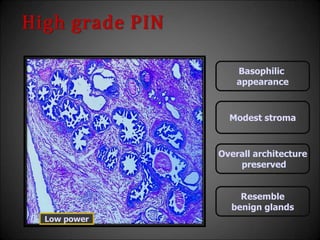
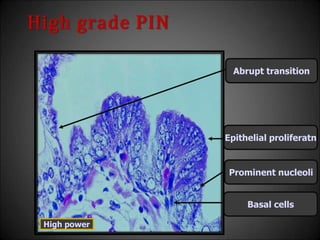
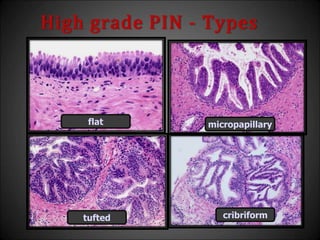
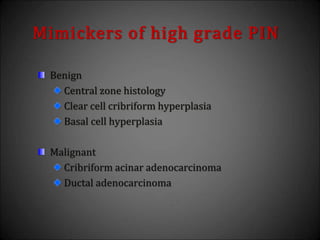
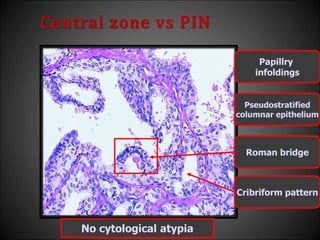
This document discusses various histological structures and lesions that can mimic prostate carcinoma on biopsy. It describes entities such as atrophy, basal cell hyperplasia, adenosis, non-specific granulomatous prostatitis, and clear cell cribriform hyperplasia that resemble low or high-grade prostate cancer. Distinguishing these mimickers from cancer relies on architectural features, cytology, immunohistochemistry, and the presence of basal cells. While some mimickers can be difficult to differentiate from cancer on limited biopsy sampling, correlation with clinical findings and use of immunohistochemical markers are important to arrive at an accurate diagnosis.




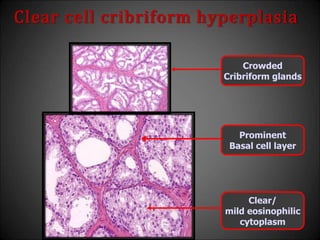
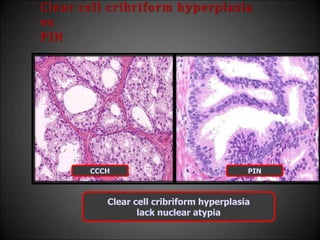
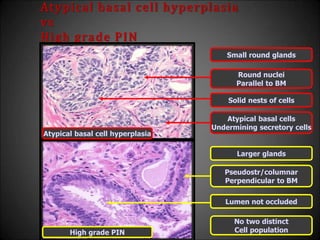
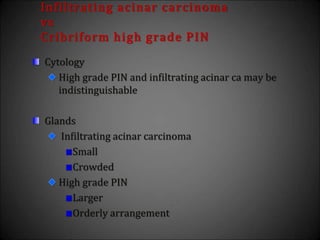

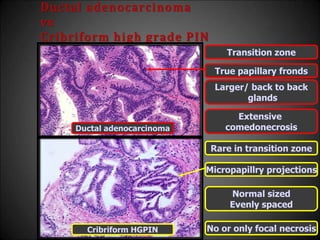










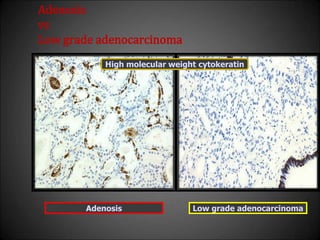
![Adenosis
vs
Low grade adenocarcinoma
• Features that do not differentiate
Back to back crowded glands
Intraluminal crystalloids
Medium sized [<3µ] nucleoli
Scattered poorly formed glands and single cells
Minimal infiltration at the periphery](https://image.slidesharecdn.com/mimickersofprostaticcarcinoma-220316163603/85/Mimickers-of-prostatic-carcinoma-29-320.jpg)
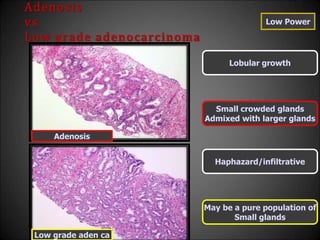
![Adenosis
vs
Low grade adenocarcinoma
High Power
Huge[>3µ] nucleoli absent
Occasionally huge nucleoli +
Nuclear and Cytopl features
same as large benign glands
Differ from surrounding
Benign glands
Pale clear cytoplasm
Amphophilic cytoplasm
Occasional glands with
Basal cells
Basal cells absent
Adenosis
Low grade aden ca](https://image.slidesharecdn.com/mimickersofprostaticcarcinoma-220316163603/85/Mimickers-of-prostatic-carcinoma-31-320.jpg)