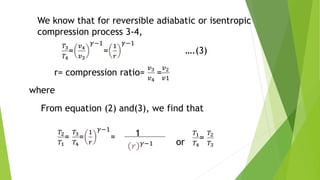
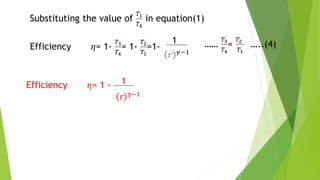
The document summarizes the Otto cycle, which is the theoretical cycle of operation for gasoline engines. It consists of four processes: (1) an adiabatic expansion of the air/fuel mixture, (2) a constant-volume heat rejection process, (3) an adiabatic compression process, and (4) a constant-volume power stroke. The efficiency of the Otto cycle is calculated based on the temperature changes during the four processes and is maximized by increasing the compression ratio. This cycle forms the basis for comparison with other internal combustion engine cycles.