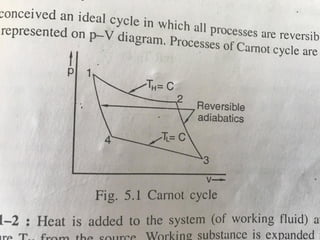
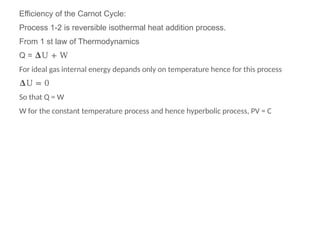
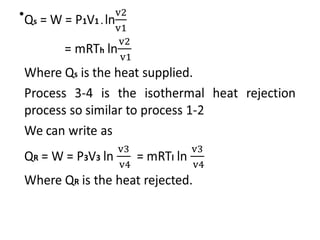
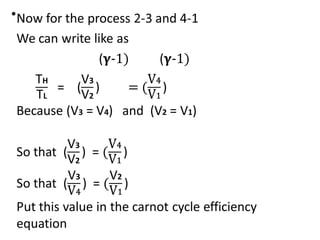
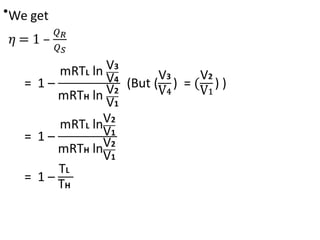
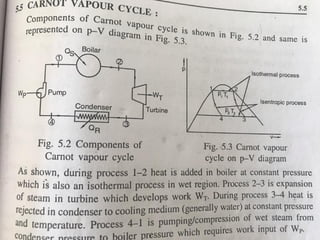
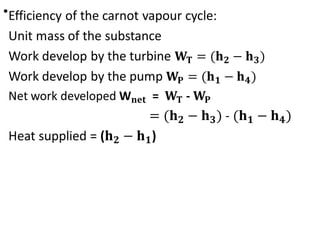
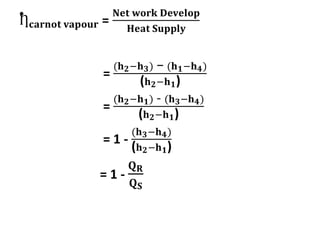
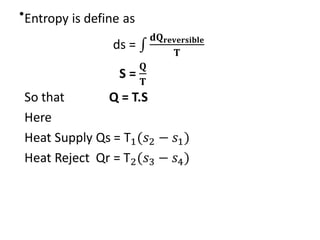
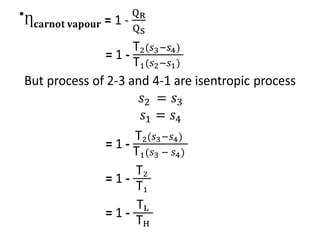
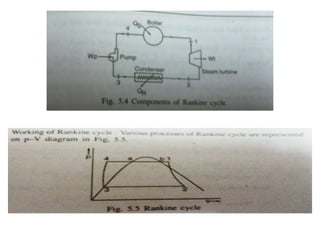
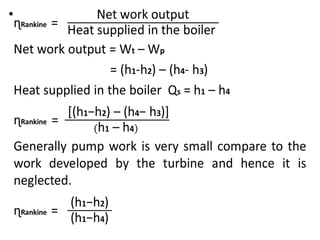
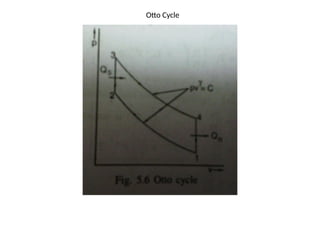
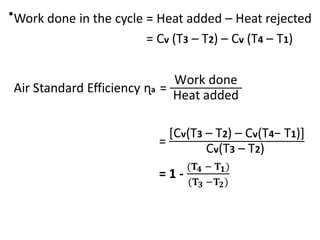
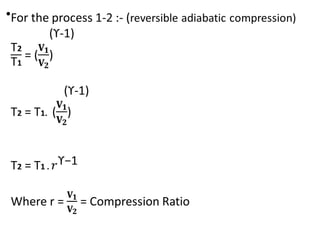
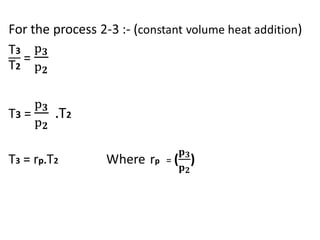
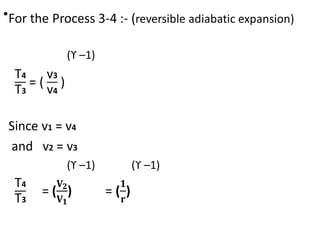
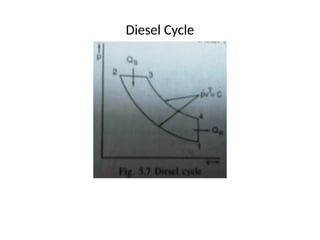
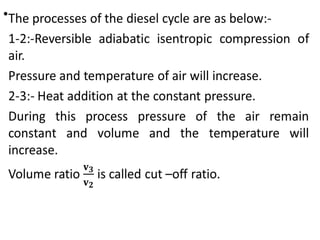
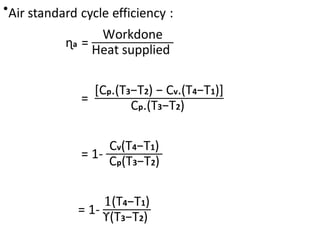
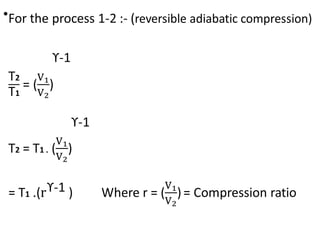
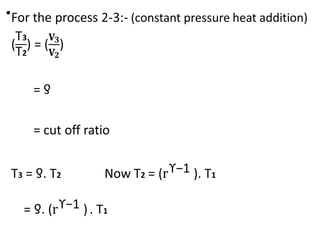
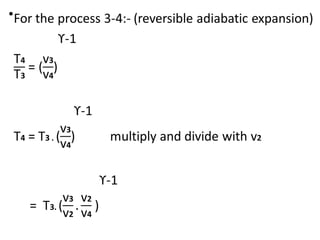
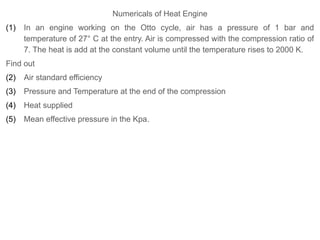
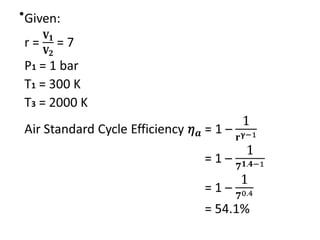
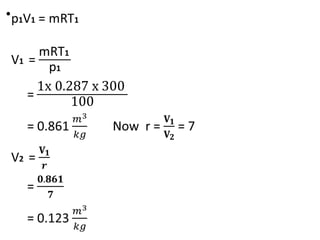
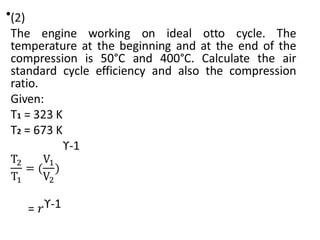
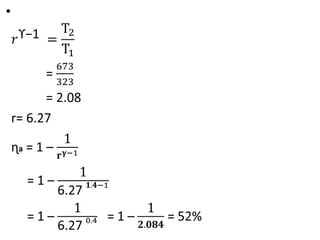
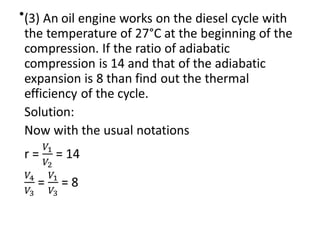
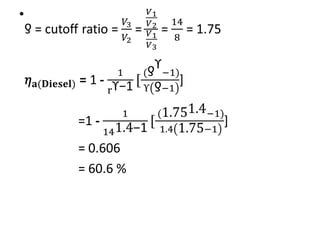
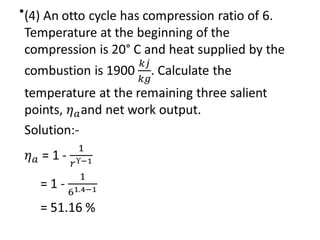
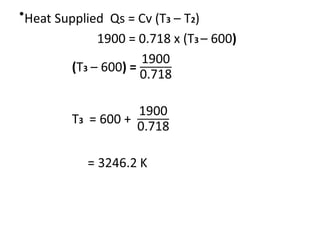
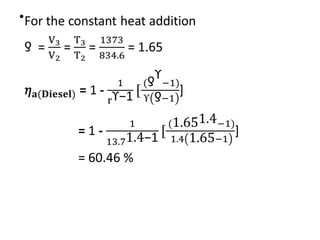
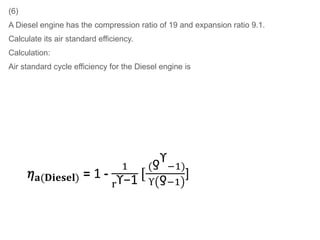
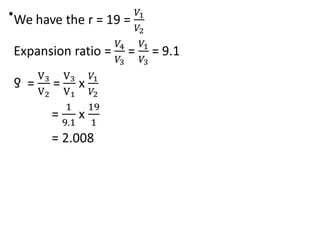
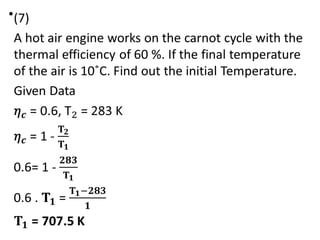
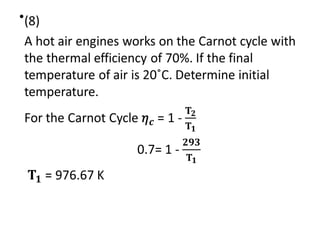
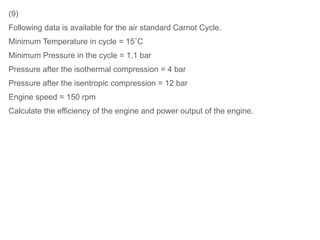
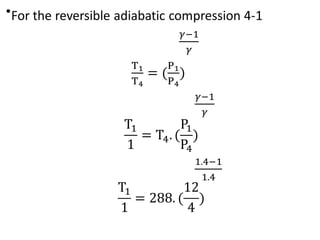
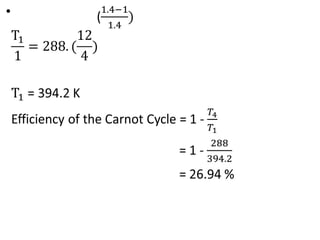
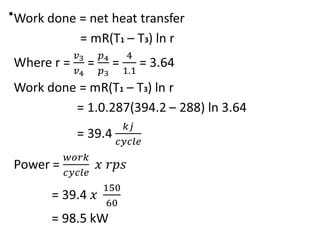
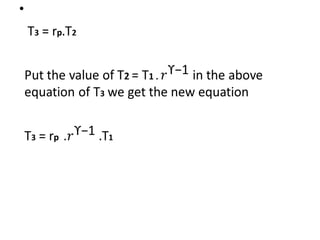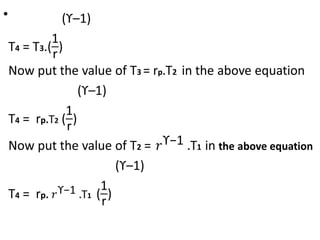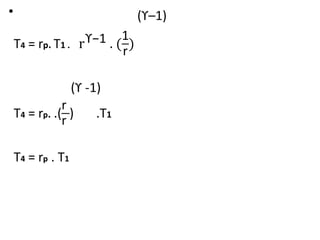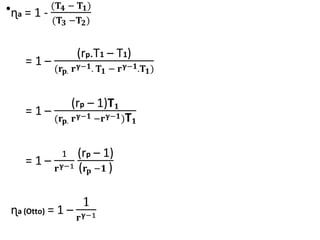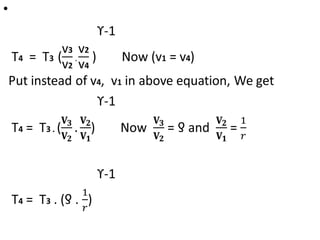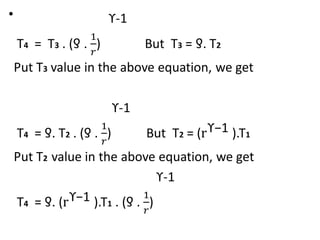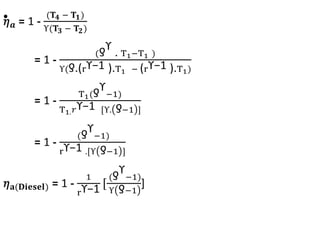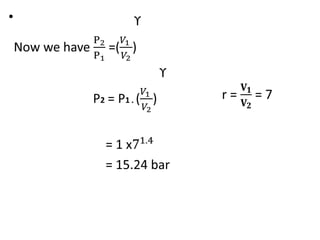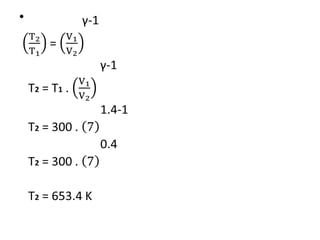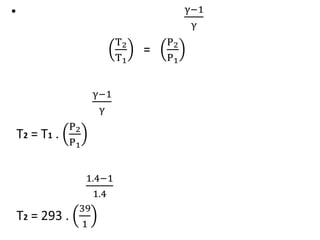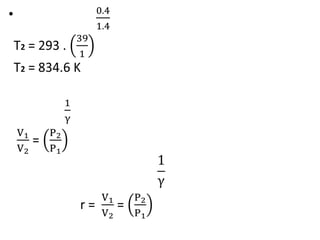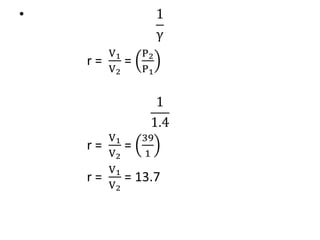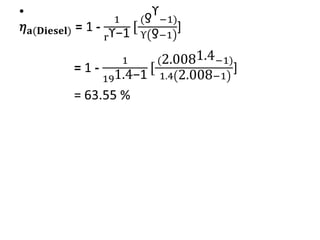The document provides an overview of heat engines, detailing their classifications into external and internal combustion engines, and the essential components involved in their operation. It outlines the various thermodynamic cycles such as Carnot, Rankine, Otto, and Diesel cycles, including their processes and efficiencies. Additionally, it discusses the characteristics and differences between internal and external combustion engines along with examples of working fluids used in these systems.