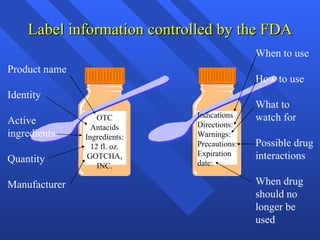
The document discusses over-the-counter (OTC) and prescription drugs. Prescription drugs require approval from a health professional, while OTC drugs can be purchased without approval. Both can be dangerous if abused or taken incorrectly. The FDA regulates OTC drug labels to provide information on active ingredients, uses, directions, warnings, and expiration dates to promote safe use. Common OTC drugs include pain relievers, cough and cold medicines, and gastrointestinal medications.