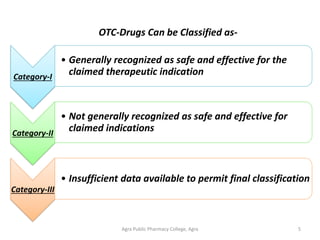
This document discusses over-the-counter (OTC) drugs, including their definition, classification, use, and regulation. It notes that OTC drugs are medicines that can be purchased without a prescription because they are considered safe and effective for self-treatment of minor ailments when used as directed. The document outlines the three phase review process by which the FDA approves OTC drug ingredients and publishes drug monographs establishing conditions for general recognition of their safety and effectiveness. It emphasizes the importance of educating consumers about the rational, responsible, and risk-aware use of OTC medications to promote safe self-treatment without medical supervision.