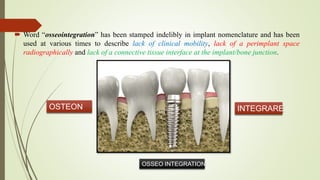
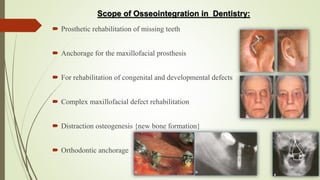
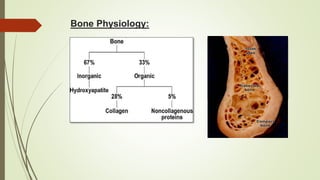
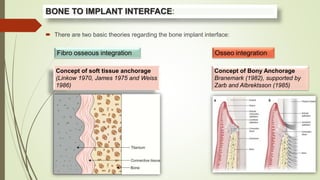
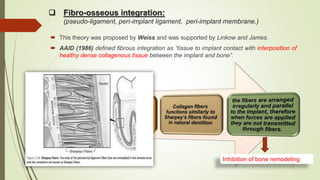
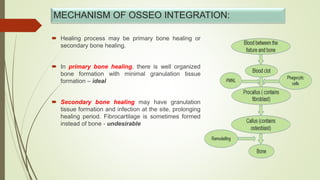
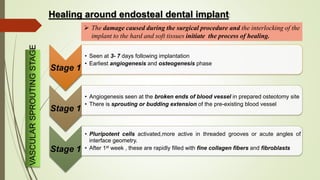
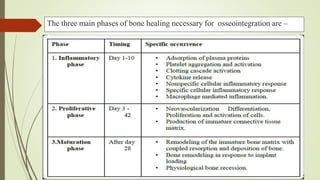
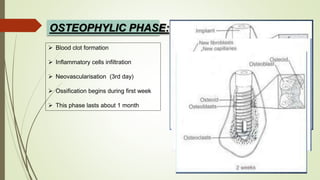
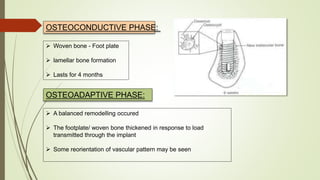
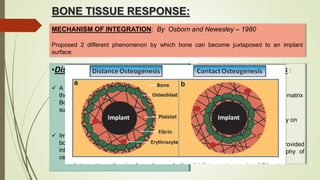
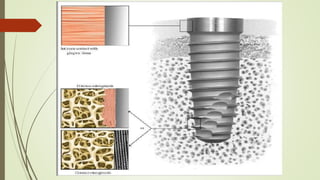
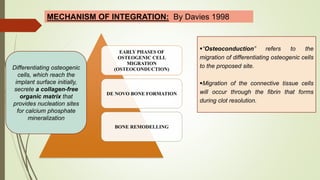
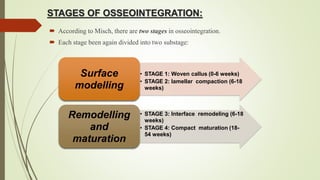
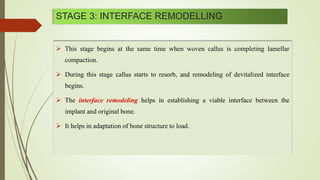
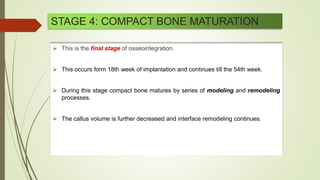
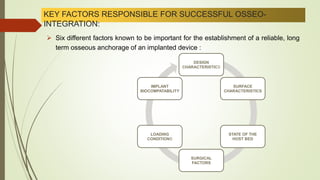
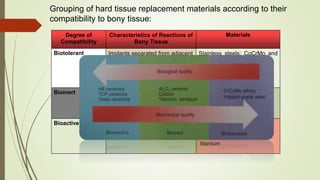
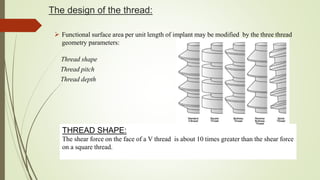
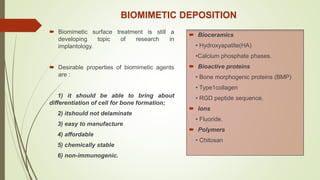
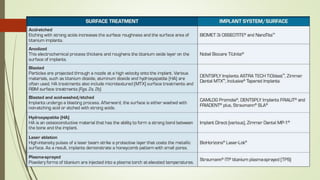
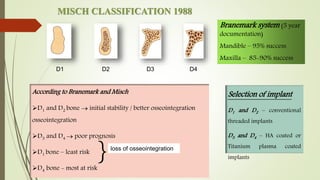
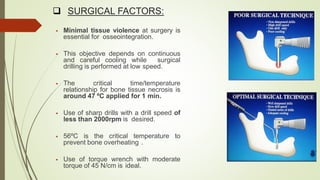
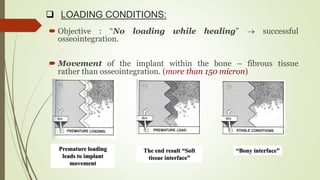
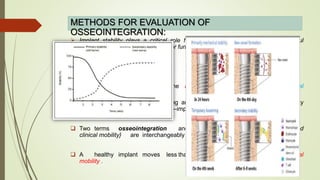
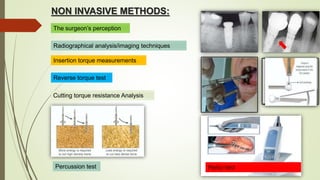
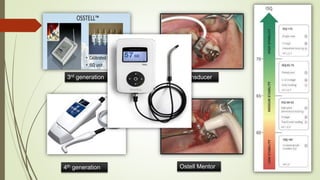
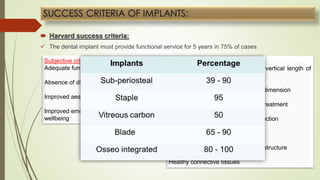
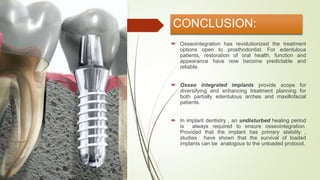
The document discusses the concept of osseointegration in implant dentistry, which is critical for the successful placement of dental implants. It outlines the historical development of osseointegration, key definitions, mechanisms, phases of healing, and factors influencing successful integration. The document also emphasizes the importance of implant design and surface characteristics in promoting osseointegration, providing a comprehensive overview of the topic.