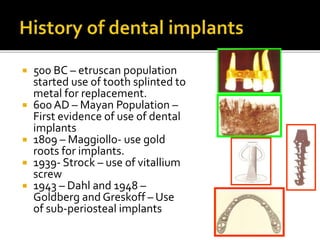
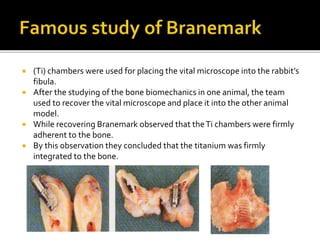
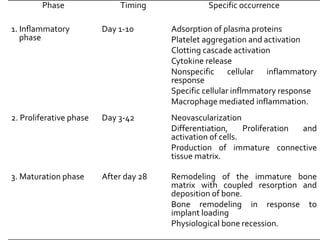
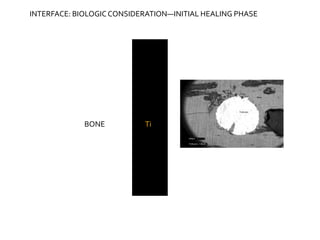
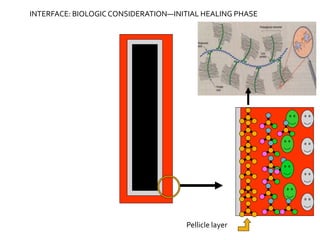
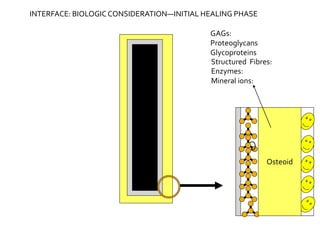
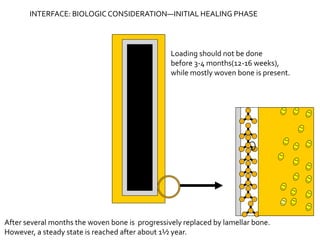
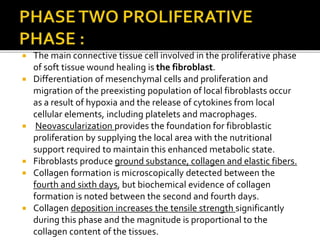
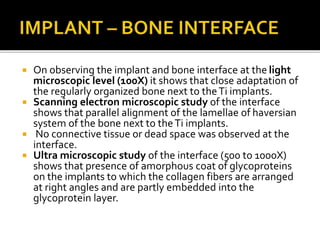
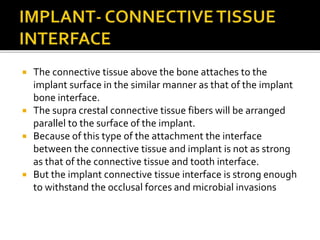
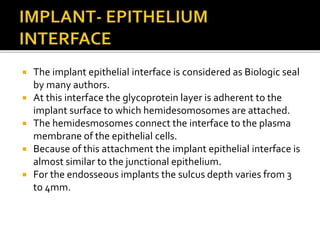
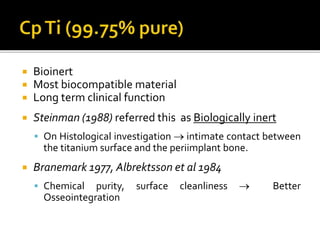
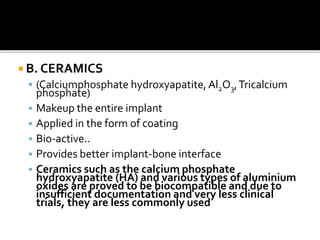
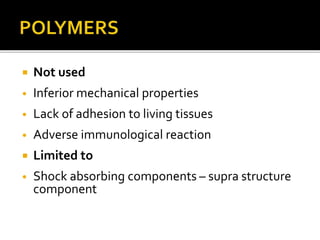
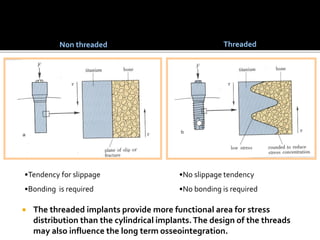
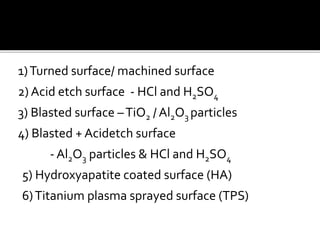
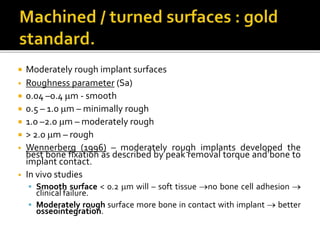
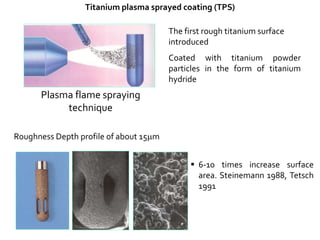
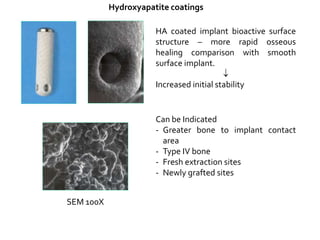
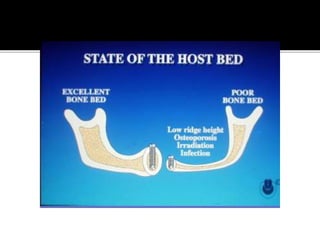
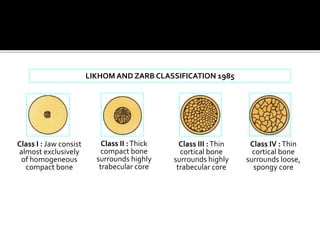
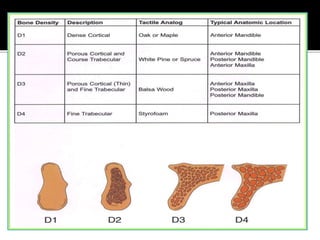
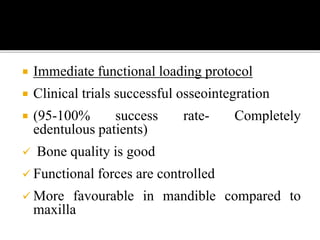
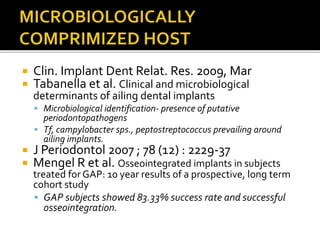
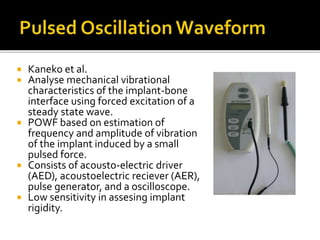
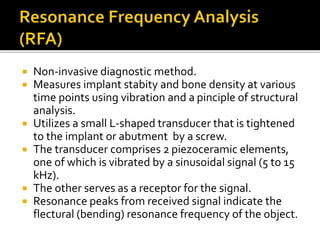
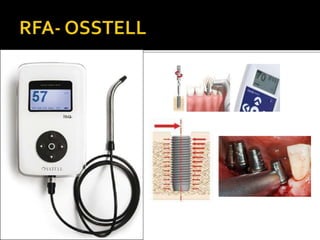
The document discusses the history and process of osseointegration of dental implants. It traces the early attempts at tooth replacement back to ancient civilizations. The concept of osseointegration was developed in the 1950s by Dr. Per Ingvar Branemark who observed firm integration between titanium chambers and bone in animal studies. This led to successful use of titanium implants for tooth replacement in humans. The document then describes the three phases of osseointegration - inflammatory, proliferative, and remodeling - and the cellular and tissue processes involved at the bone-implant interface during healing.