- Osmosis is the diffusion of solvent through a semi-permeable membrane from a lower to higher solute concentration until equilibrium is reached. Tonicity refers to the osmotic pressure between two solutions separated by a membrane.
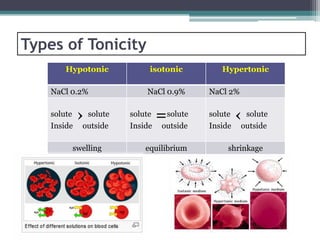
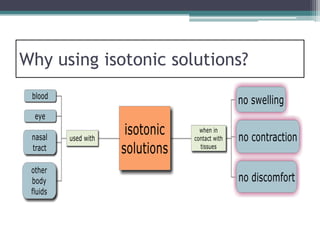
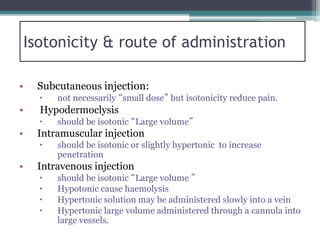
- There are three types of tonicity: hypertonic, isotonic, and hypotonic. Solutions used for administration should be isotonic to avoid cell shrinkage or swelling.
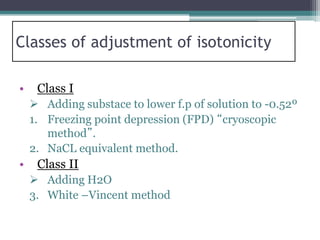
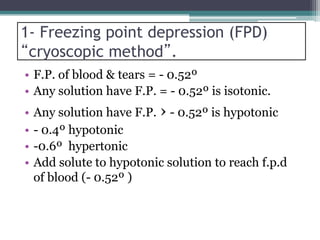
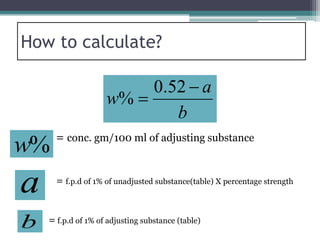
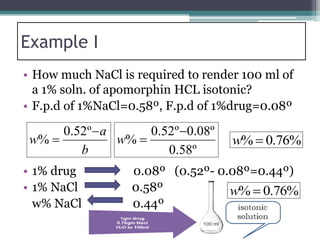
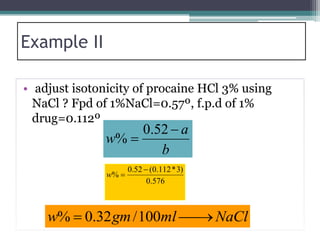
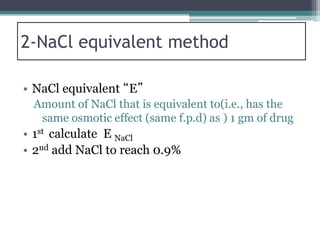
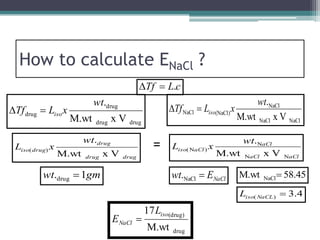
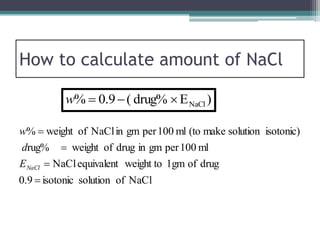
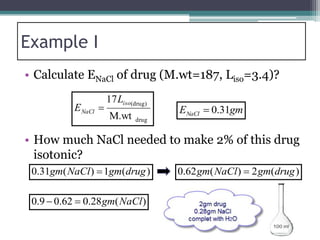
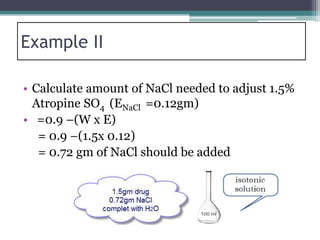
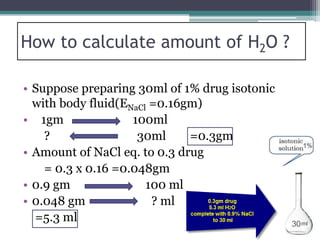
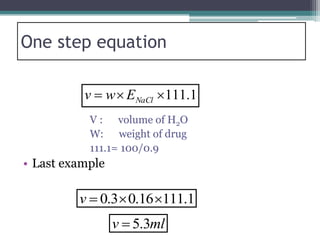
- There are several methods to adjust tonicity including calculating freezing point depression, determining NaCl equivalence, and using the White-Vincent method of first adding water then completing the volume with isotonic solution.

![osmosis
• Osmosis is the diffusion of solvent through a semi-
permeable membrane.
▫ Water always flows from lower solute concentration
[dilute solution] to higher solute concentration until a
balance is produced
• Osmotic pressure is the force that cause this
diffusion .
• Tonicity is a measure of the
osmotic pressure of two solutions
separated by a semi-permeable
membrane.](https://image.slidesharecdn.com/isotonicity-230301124428-b9fa9e59/85/Isotonicity-ppt-2-320.jpg)



![II
example
Add volume of H2O and then complete with
isotonic solution
Phenacaine HCl 0.06 gm (ENaCl=0.16)
Boric acid 0.3 gm (ENaCl=0.5)
sterile distilled H2O up to 100 ml
V = 111.1 x(weight x ENaCl)
V =111.1 x [(0.06x0.16)+(0.3x0.5)] = 17.7 ml H2O](https://image.slidesharecdn.com/isotonicity-230301124428-b9fa9e59/85/Isotonicity-ppt-21-320.jpg)