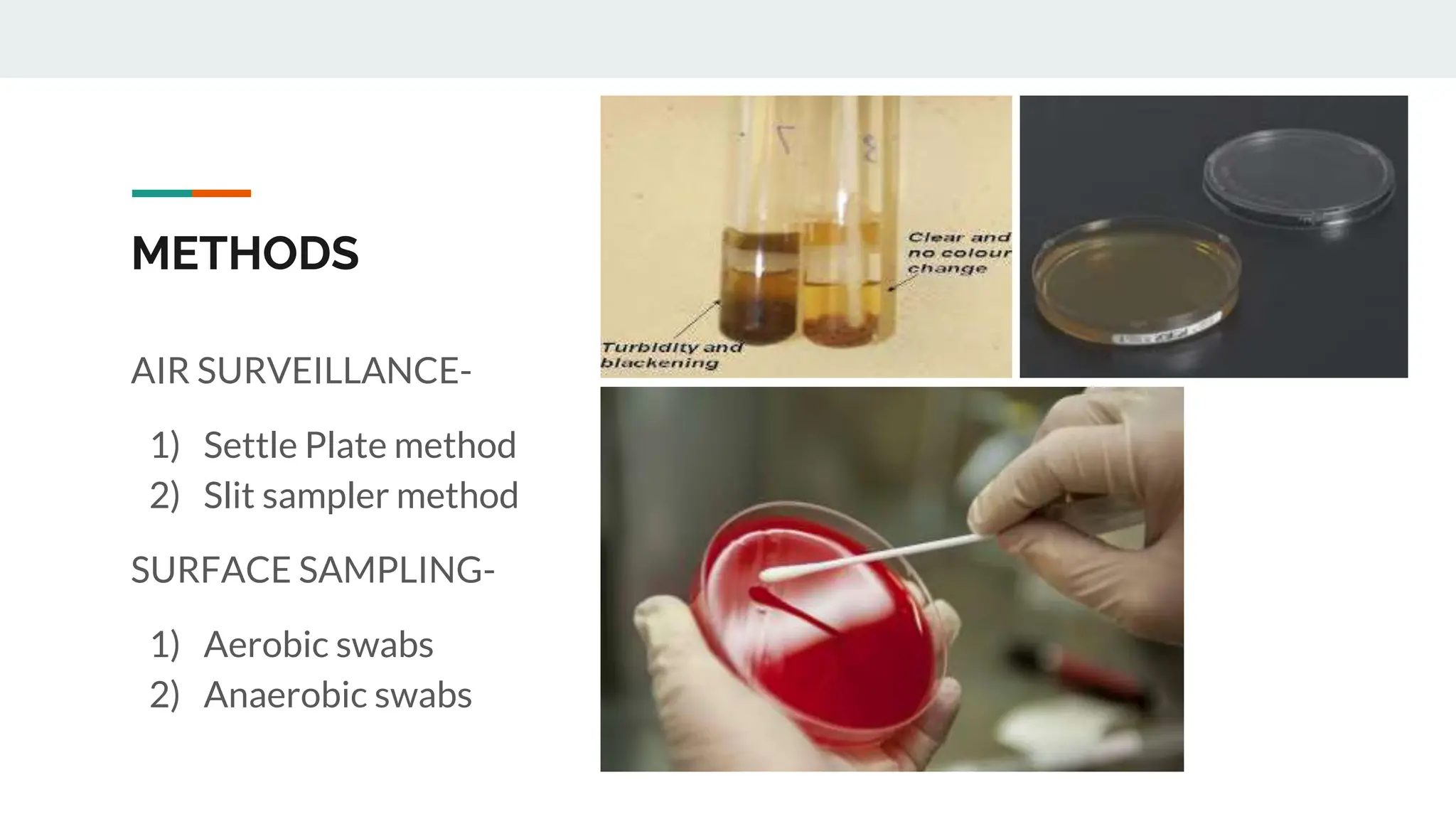
The document summarizes methods for sterility testing in operation theatres, focusing on air surveillance and surface sampling techniques. Key methods include the settle plate method and slit sampler method to monitor microbial contamination levels and ensure hygienic conditions. Results indicate that effective monitoring and adherence to recommended bio load limits are essential for controlling nosocomial infections in critical care areas.