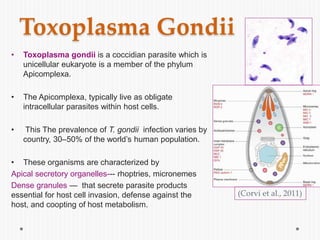
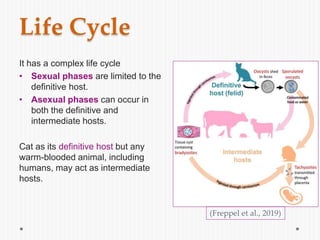
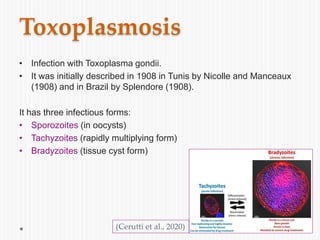
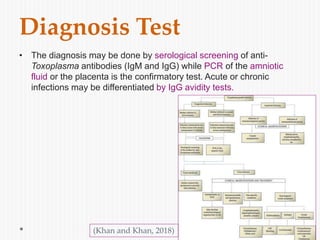
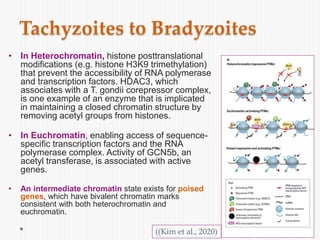
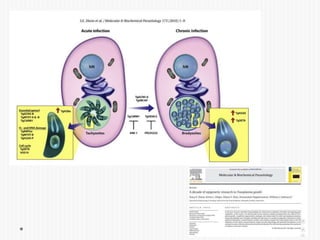
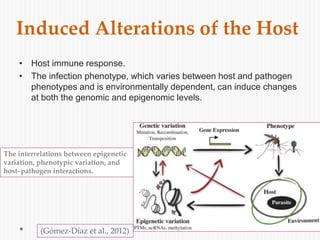
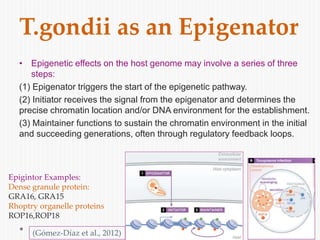
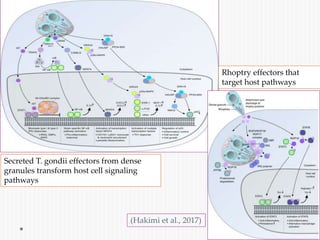
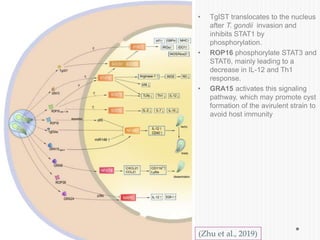
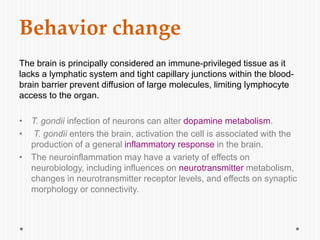
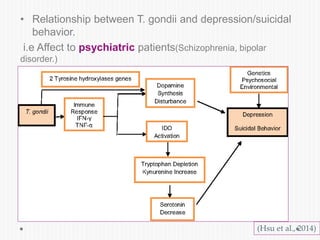
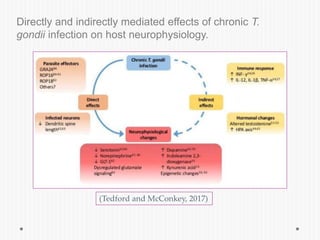
The document discusses Toxoplasma gondii, a unicellular parasite with a complex life cycle that can infect humans, leading to toxoplasmosis. This infection can have congenital effects, neurological implications, and influence host behavior, particularly in terms of immune response and neurophysiology. It highlights the significance of epigenetic changes in both the parasite and the host, and suggests that preventing infection, especially in cats, could reduce transmission risks.