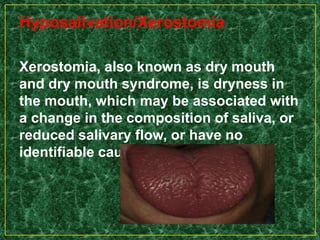
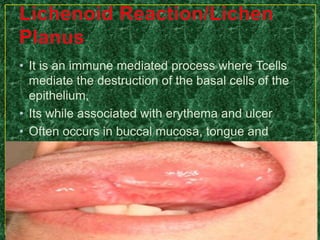
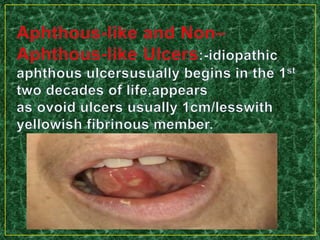
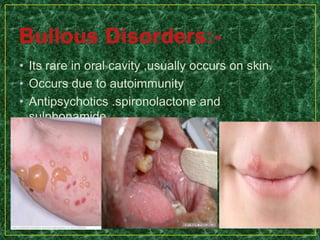
This document discusses various methods for pharmacological and toxicological screening of substances. It provides details on:
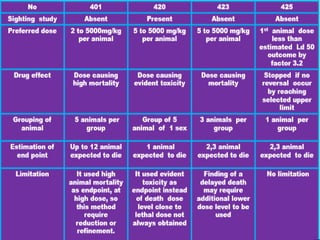
- Types of toxicity testing including acute, sub-acute, sub-chronic, and chronic toxicity tests.
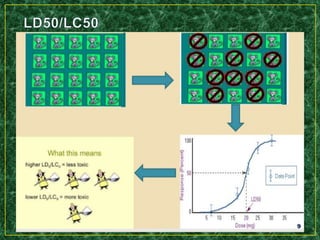
- Methods for determining lethal dose 50 (LD50) and lethal concentration 50 (LC50) values which represent doses/concentrations that are lethal to 50% of test subjects.
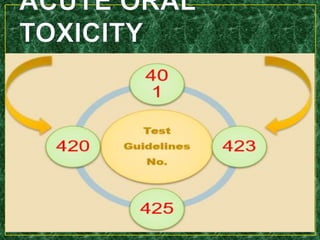
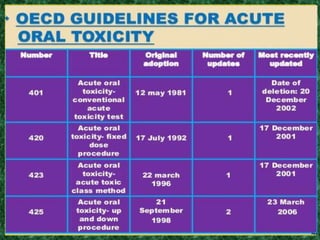
- Specific acute oral toxicity testing methods like Karber's method, Miller-Tainter method, and up-and-down method.
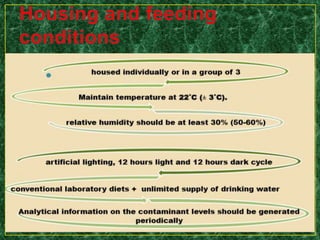
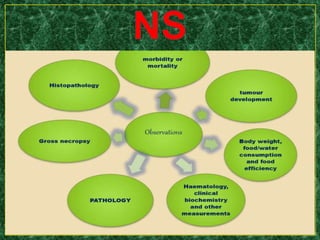
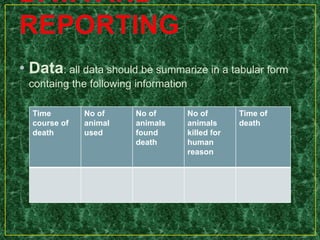
- Testing procedures which involve administering graduated doses of a test substance to rodents and making observations to determine health hazards.



























![• Its calculate using likelihood method ,
following dixion .assume 𝜕 as death or
delay death
• L=L1L2………Ln
• Li=1-f(Zi) if animal survives
• Li =f(zi) if animal dies
• Where Zi=⟮log (di)- µ ] ̸ 𝜕 di]](https://image.slidesharecdn.com/oraltoxicity-180417063442/85/Oral-toxicity-36-320.jpg)