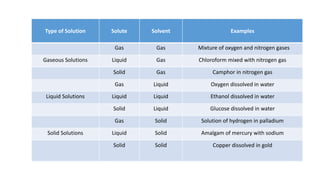
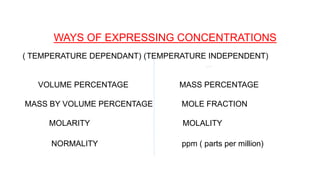
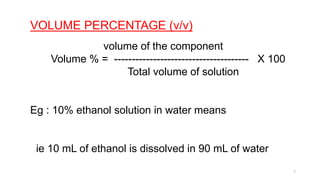
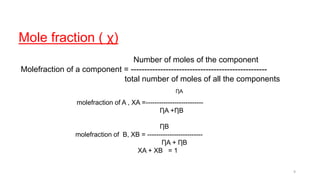
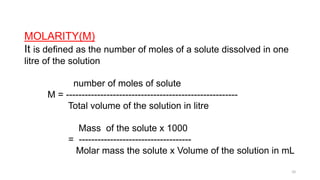
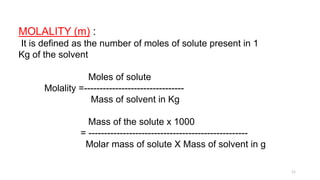
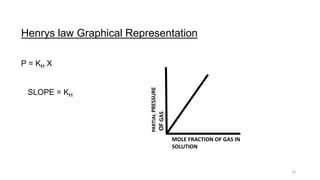
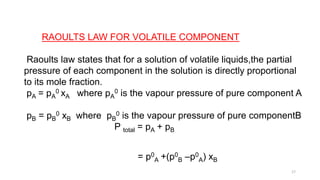
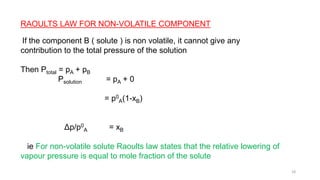
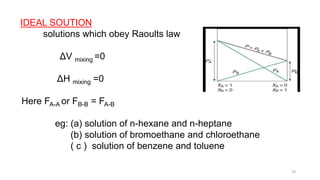
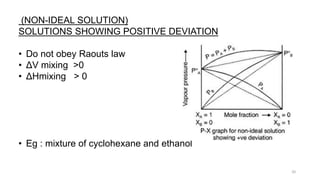
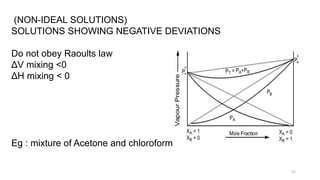
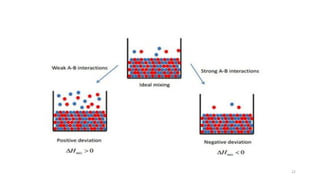
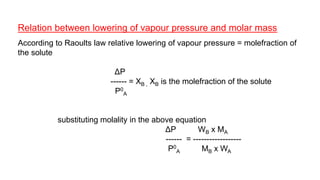
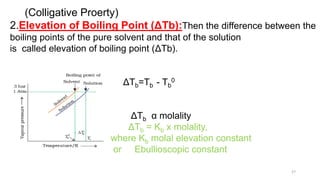
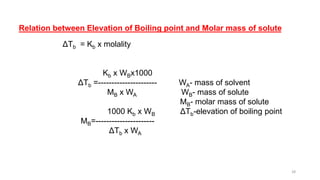
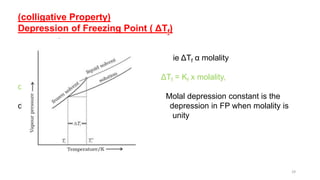
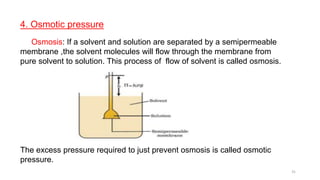
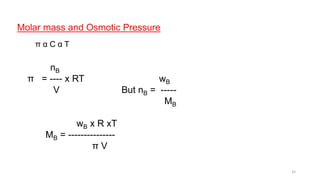
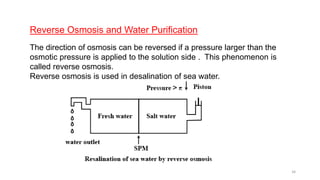
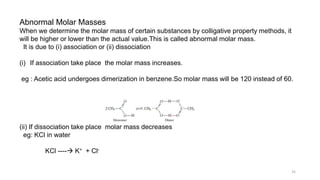
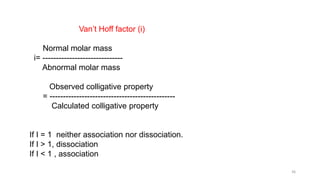
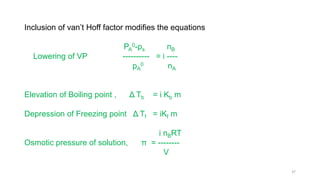
This document provides information about solutions and various methods of expressing concentration of solutions. It defines key terms like solvent, solute, binary solutions, etc. It describes different units of concentration like mass percentage, mole fraction, molarity, molality, etc. and provides examples of their calculation. It also discusses concepts like solubility, Henry's law, Raoult's law, ideal and non-ideal solutions, and colligative properties related to vapor pressure, boiling point, freezing point and osmotic pressure. It explains how these properties depend on molar mass and provides their relationships. It also touches upon abnormal molar masses due to association or dissociation and the van't Hoff factor.