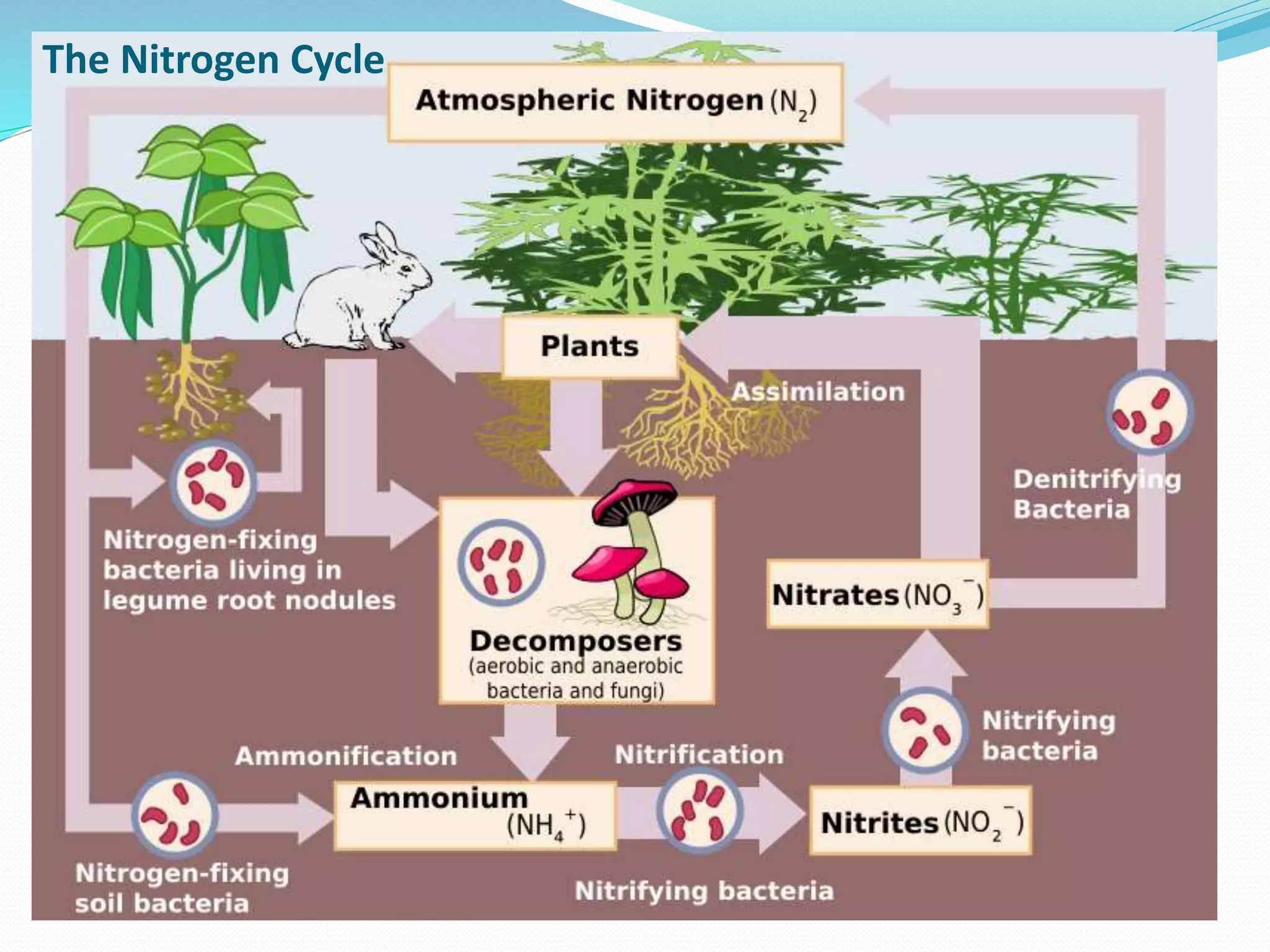
Nitrogen is abundant in the atmosphere but not directly usable by plants and animals. The nitrogen cycle describes the biological and chemical processes by which nitrogen is converted between its various chemical forms. These include nitrogen fixation by bacteria, nitrification by other microbes, assimilation by plants, ammonification through decomposition, and denitrification by some bacteria. Human activities like burning fossil fuels, livestock waste, and fertilizer use introduce additional reactive nitrogen into ecosystems, disrupting the natural cycle.