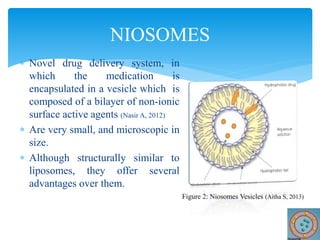
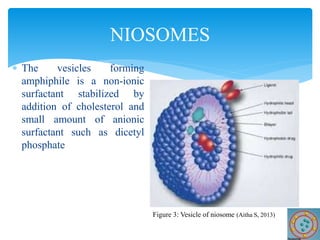
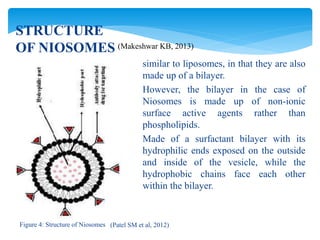
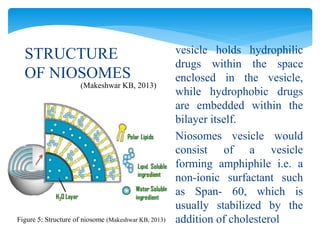
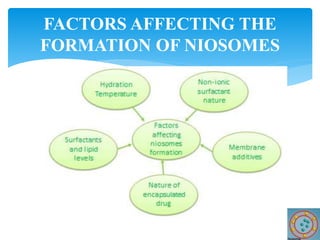
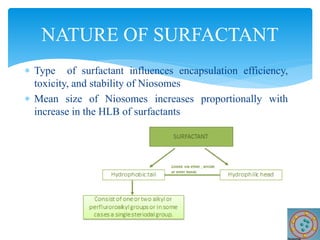
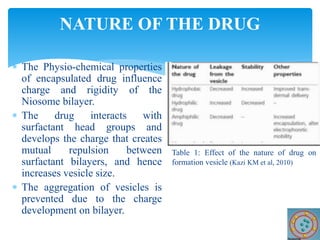
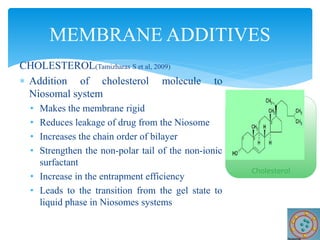
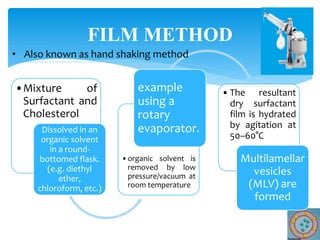
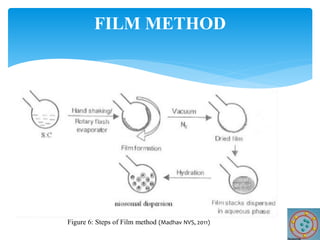
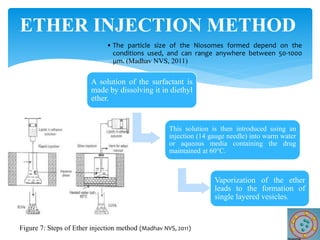
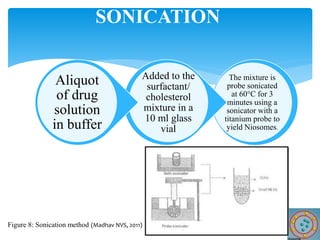
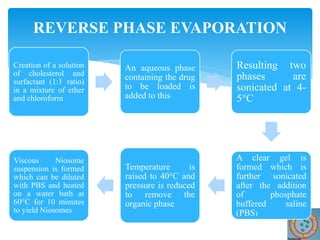
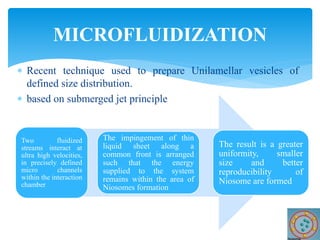
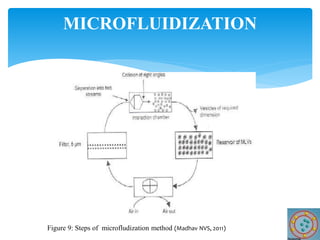
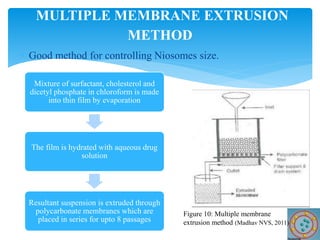
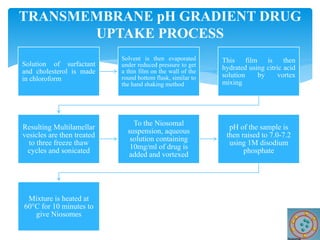
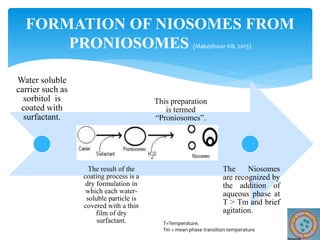
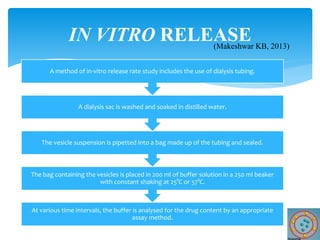
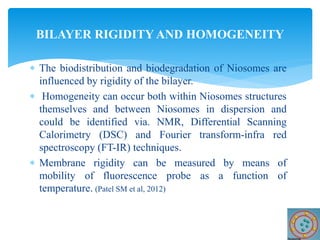
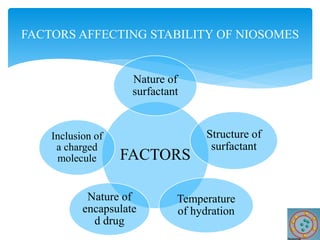
Niosomes are novel drug delivery vesicles composed of non-ionic surfactants and cholesterol. They can entrap both hydrophilic and hydrophobic drugs and offer advantages over liposomes such as improved stability and lower costs. Niosomes are formed via various methods that produce multilamellar or unilamellar vesicles of different sizes ranging from 100nm to 5um. Key factors affecting niosome formation include the type of surfactant, surfactant/lipid ratio, drug properties, addition of cholesterol, and preparation method used. Niosomes can improve drug bioavailability, target drug delivery, and provide protection from degradation.