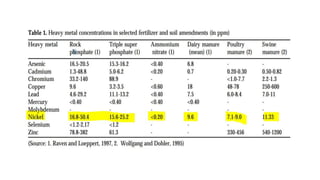
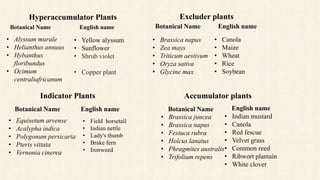
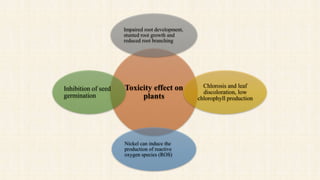
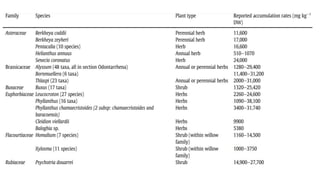
Nickel is a naturally occurring element that is found in soil, water, air, and food. It can enter the environment through natural processes like volcanic eruptions or weathering of nickel-containing rocks, as well as anthropogenic activities like mining, fossil fuel combustion, and waste incineration. Nickel is present in varying concentrations in different types of soils depending on their geological composition. In plants, nickel is taken up as Ni2+ and concentrations can vary significantly depending on plant species and soil nickel levels. While not essential in all plants, nickel plays a role in certain enzyme functions in some species. High levels of nickel exposure through food and water can cause toxicity effects in humans and animals. Phytoremediation