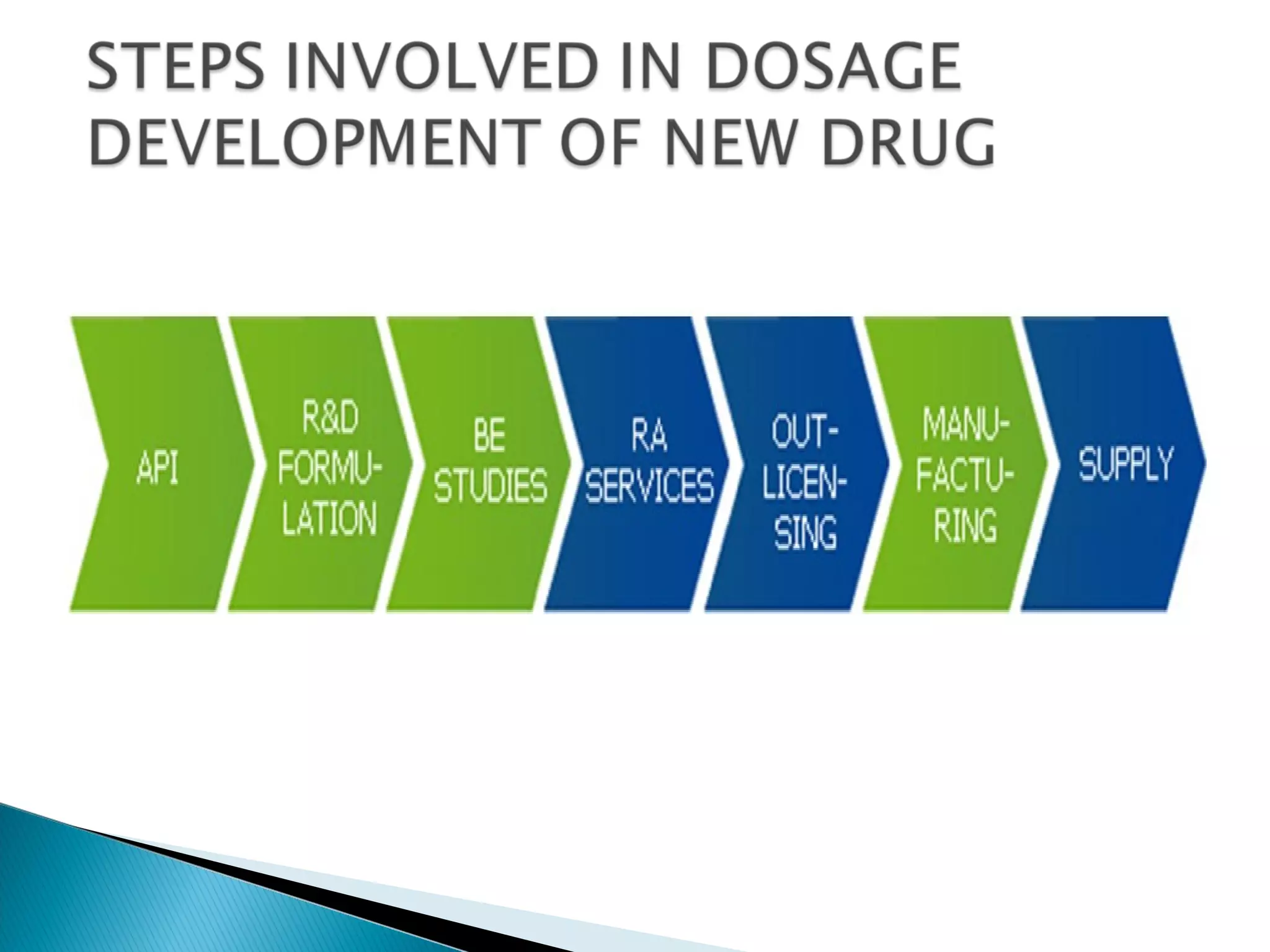
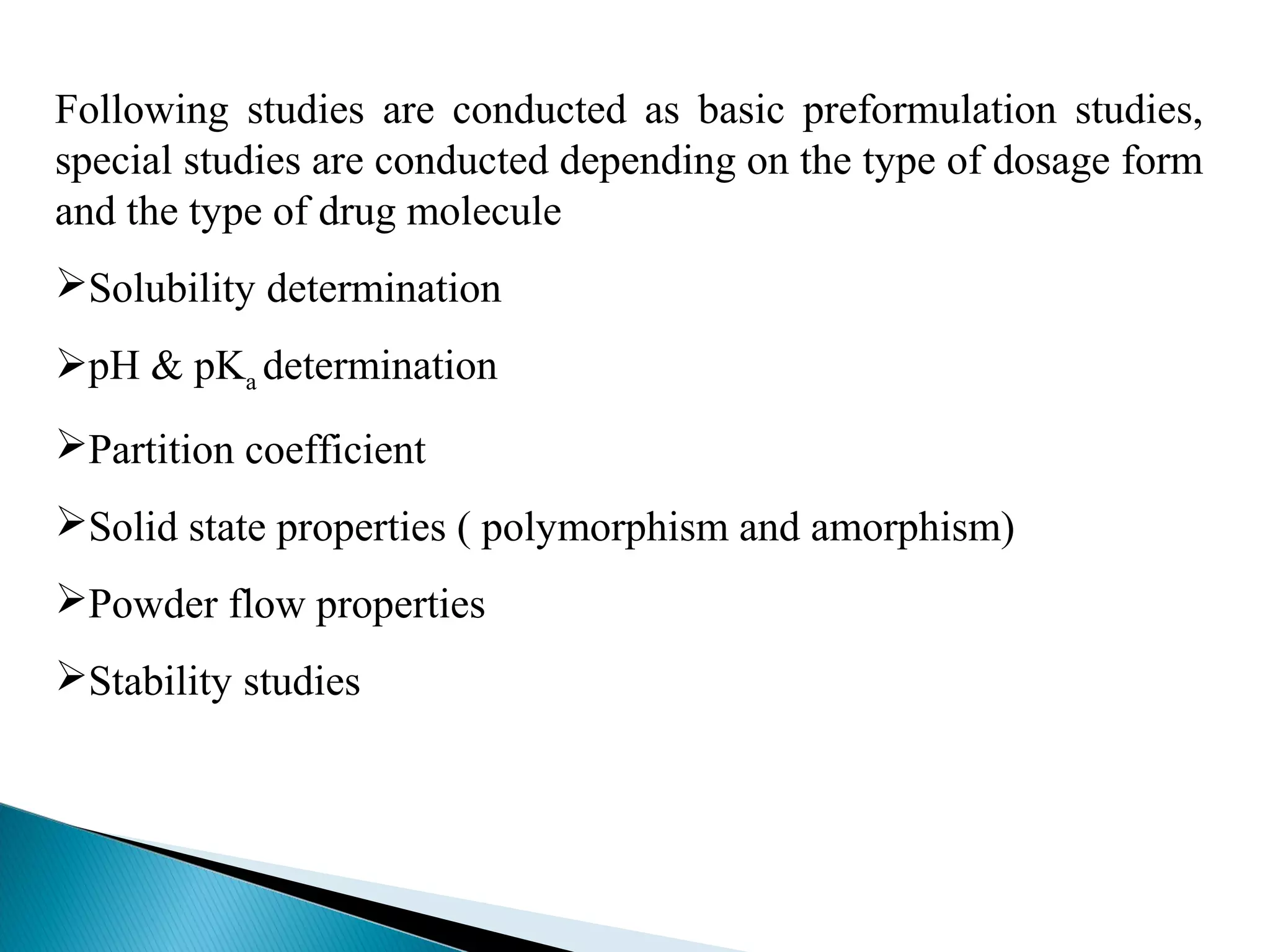
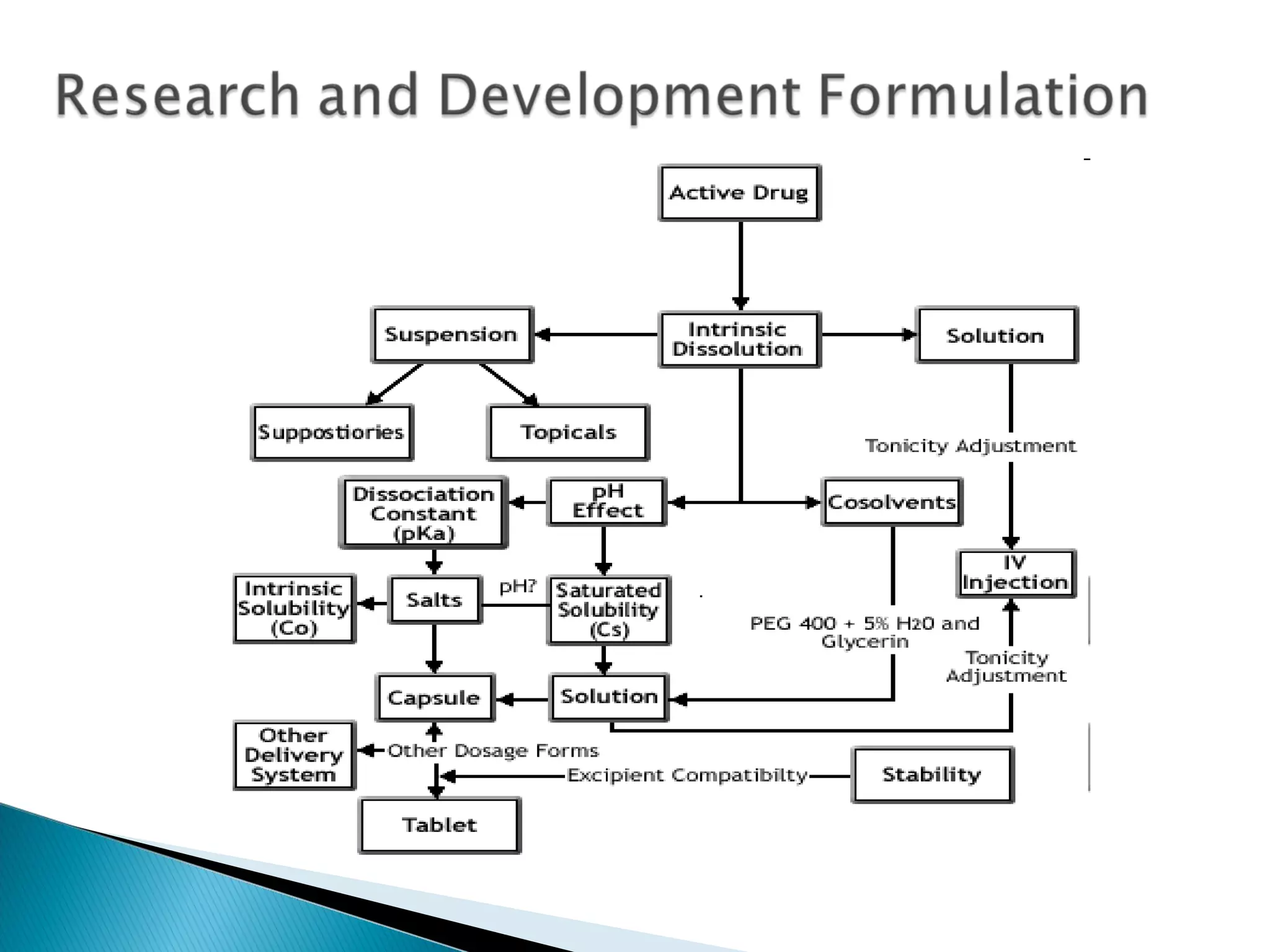
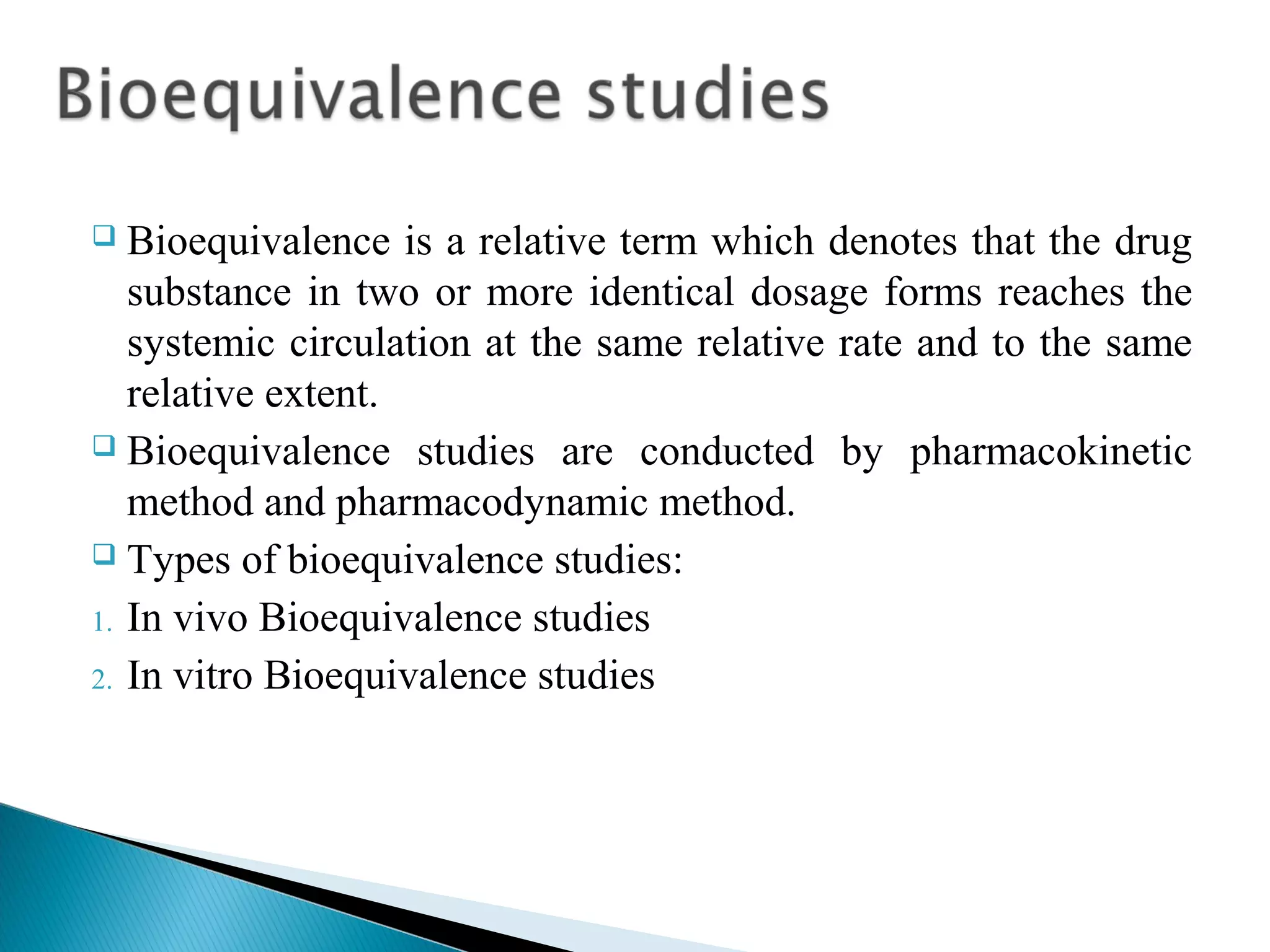
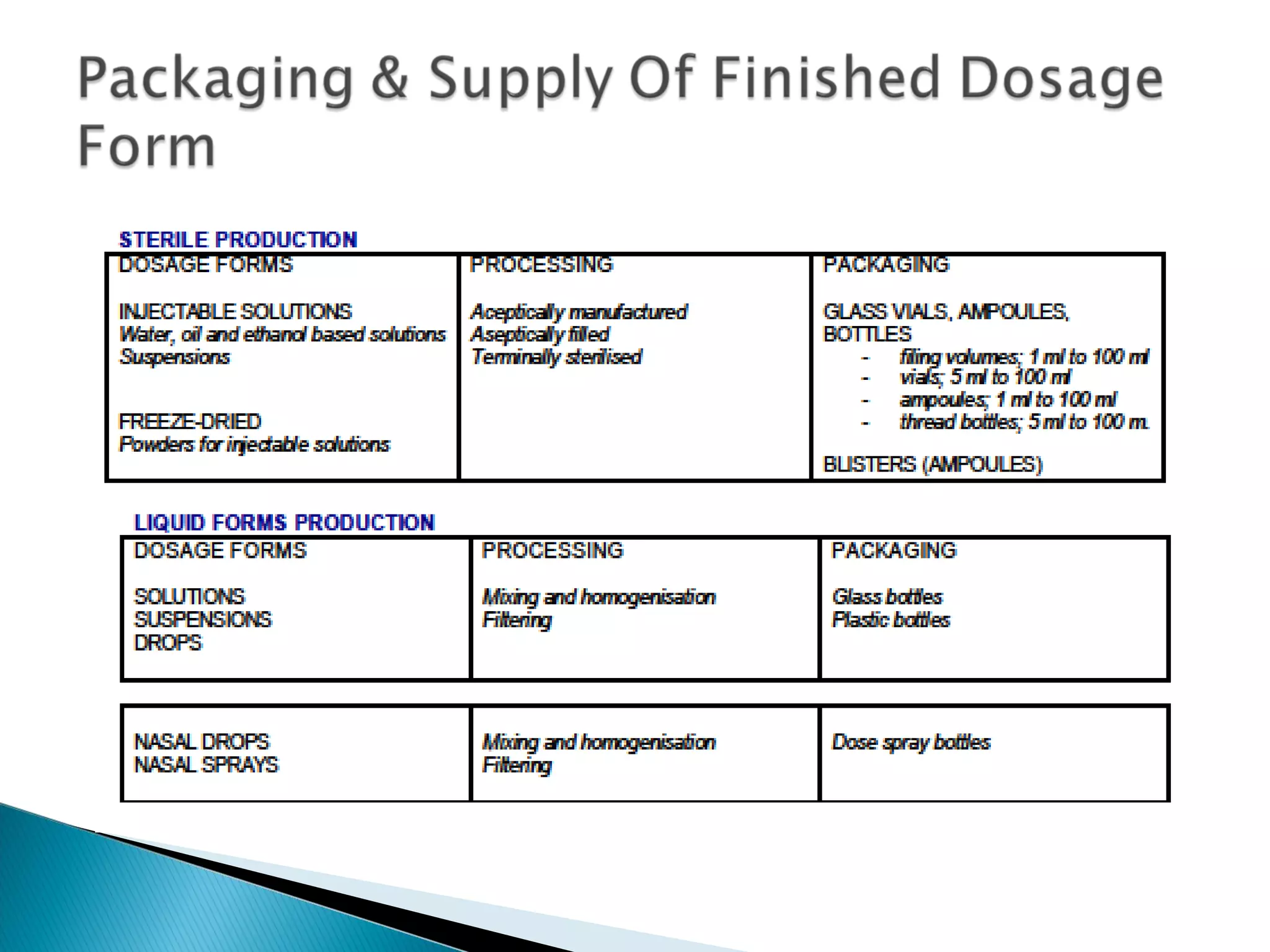
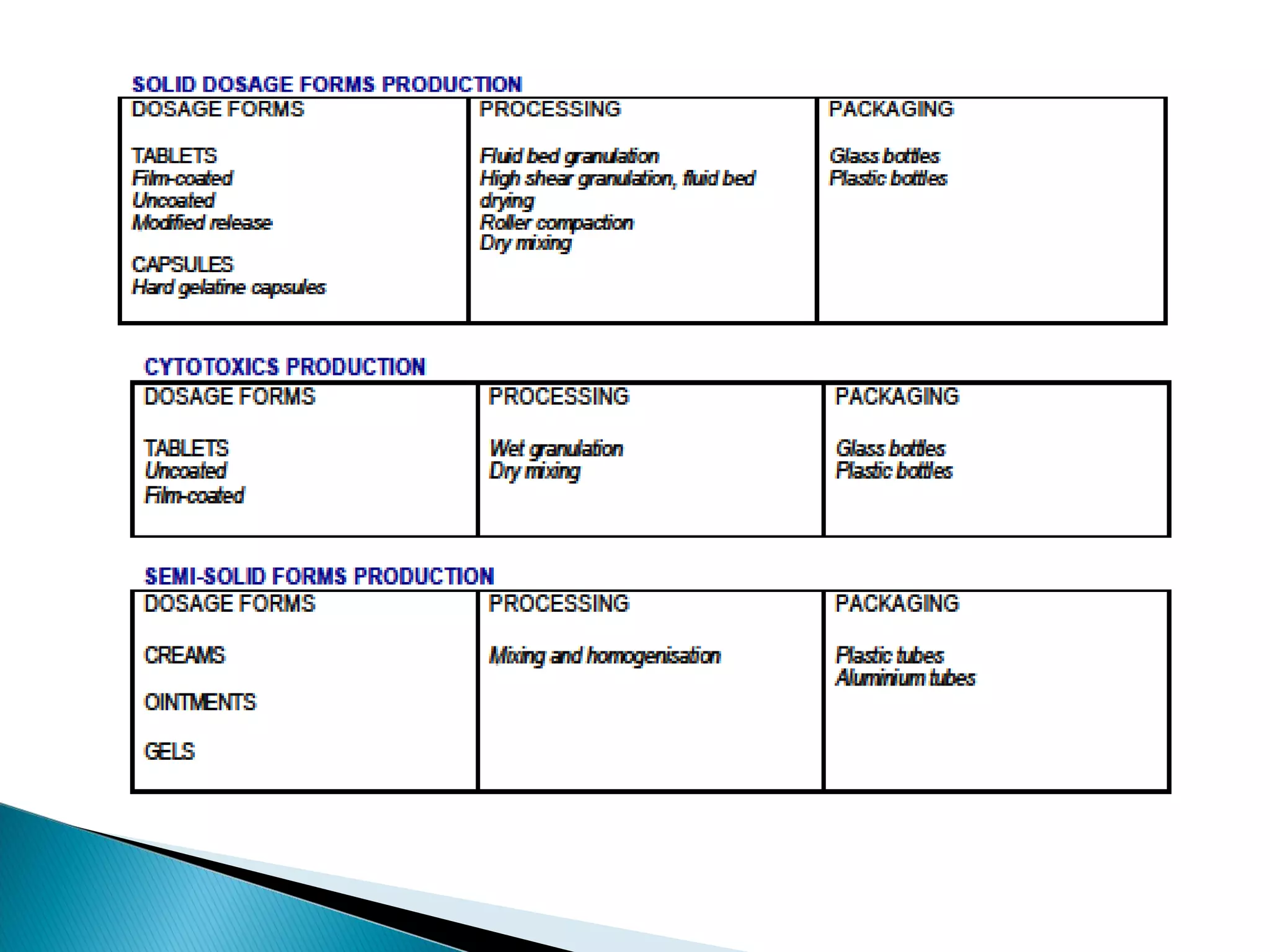
This document discusses key aspects of preformulation and formulation development. It begins with definitions of important terms like drug, dosage form, dose, and bioavailability. It then describes the basic preformulation studies conducted like solubility, pH, and stability testing. The document outlines the main components of a dosage form including active drug and excipients. It provides examples of various dosage forms like tablets, capsules, liquids, semisolids, and inhalations. Finally, it stresses the importance of following formulation development steps to create an efficient dosage form that is safe, effective and has minimal toxicity.