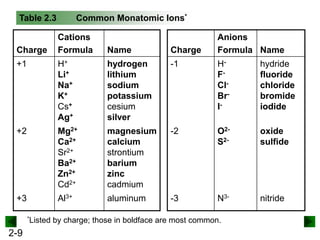
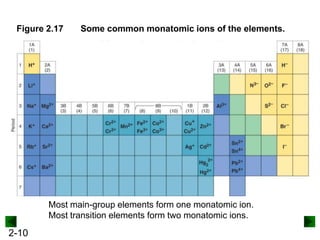
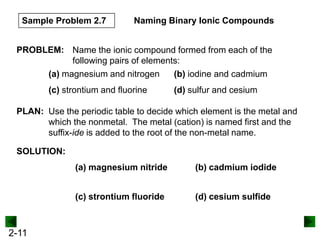
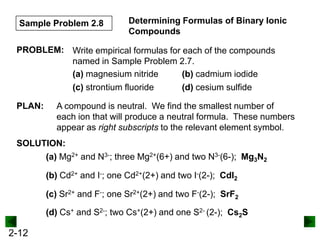
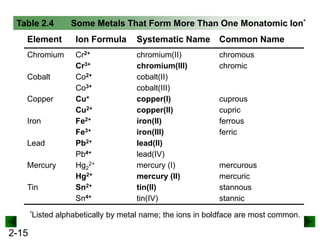
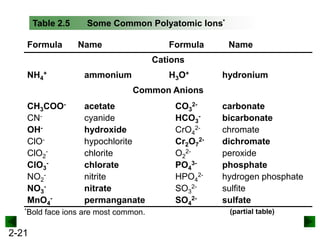
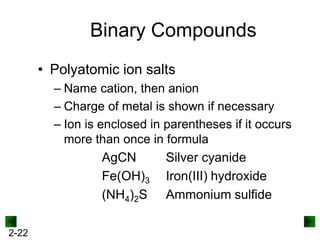
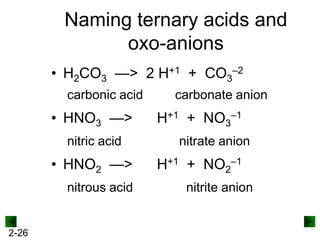
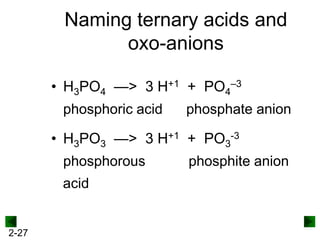
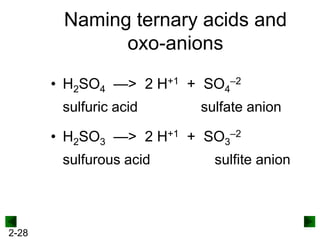
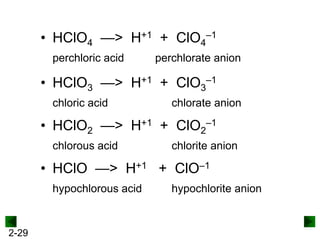
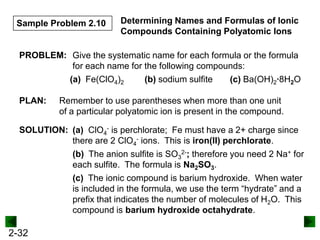
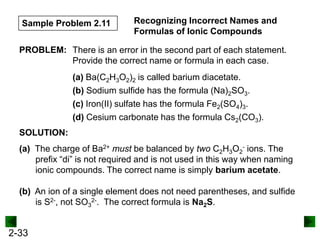
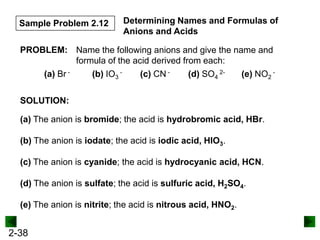
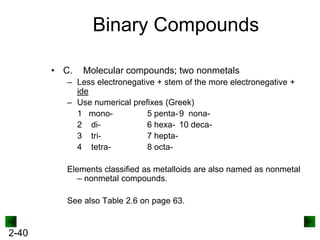
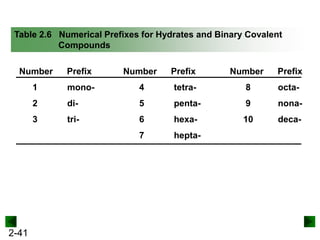
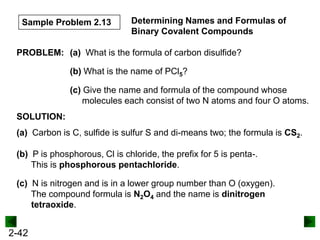
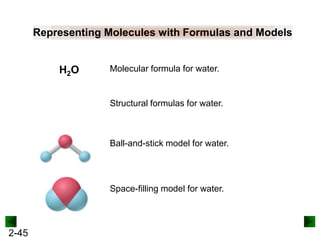
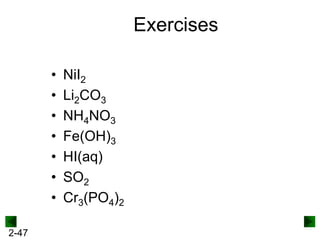
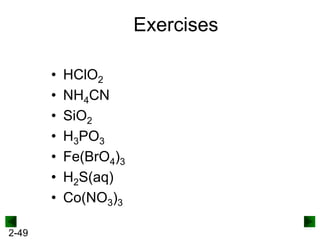
This document provides information on naming and writing formulas for inorganic compounds. It begins by discussing binary ionic compounds formed from a metal and nonmetal. It explains that the name of the cation (metal) comes first, followed by the anion (nonmetal) with the "-ide" suffix. Examples of naming compounds of common metals like calcium, magnesium, and sodium are provided. The document then discusses polyatomic ions and compounds containing them. It also covers compounds where the metal forms more than one ion. Finally, it discusses acids and naming compounds containing oxoanions. In summary, the document outlines the system for systematically naming inorganic compounds based on their formulas as well as writing formulas from IUPAC names.