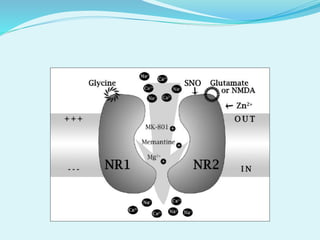
This document discusses potential neuroprotective strategies for glaucoma. It begins by explaining that glaucoma involves the loss of retinal ganglion cells and their axons, and that while elevated intraocular pressure is a risk factor, progression can still occur when pressure is lowered. Neuroprotection aims to protect these cells from degeneration. Several potential neuroprotective approaches are then described, including NMDA antagonists to reduce excitotoxicity, anti-apoptotic agents to block cell death pathways, currently used drugs that may have neuroprotective effects, antioxidants to reduce free radicals, calcium channel blockers, neurotrophic factors, gene therapy targeting apoptotic factors, and immunomodulators.