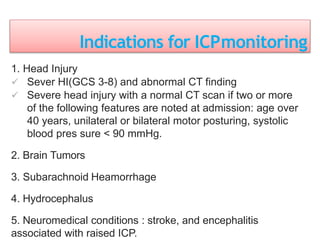
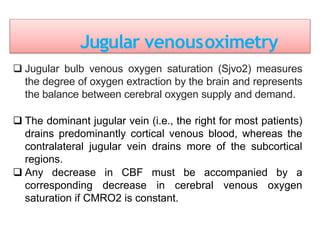
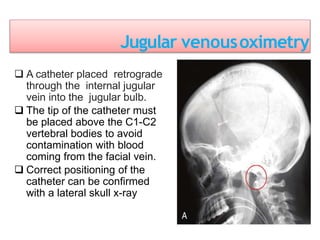
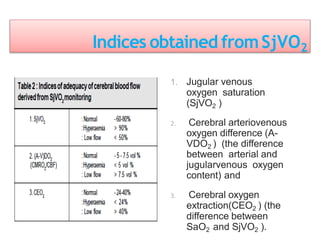
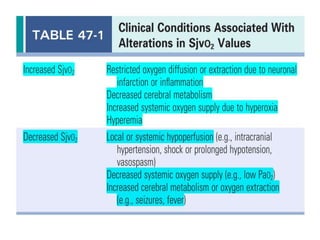
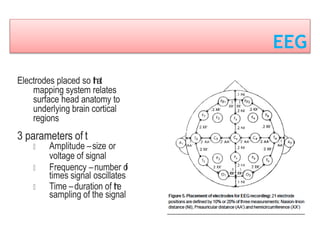
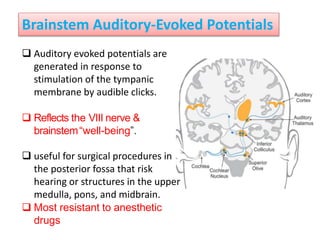
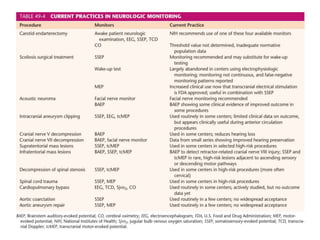
This document discusses various techniques used to monitor brain function during anesthesia, including:
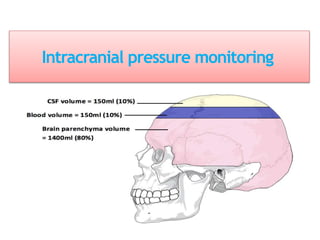
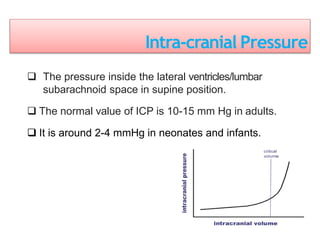
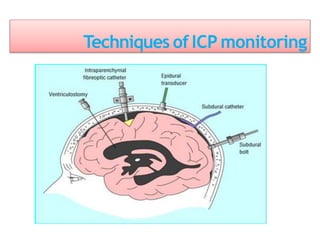
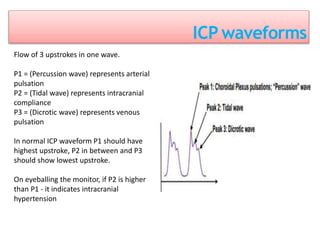
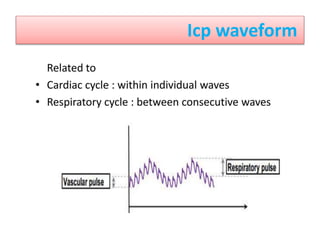
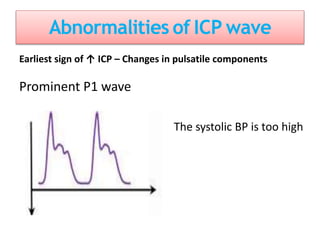
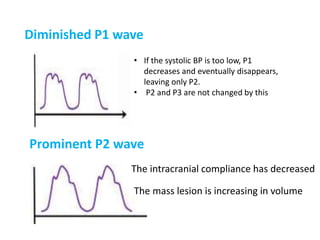
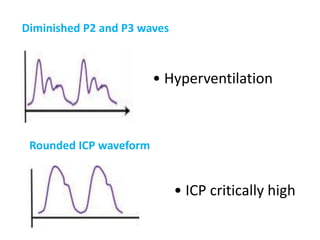
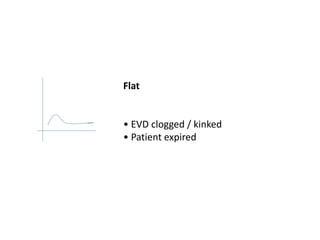
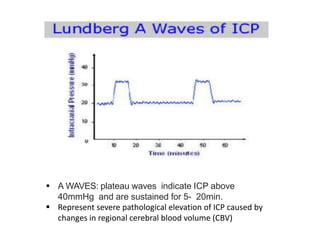
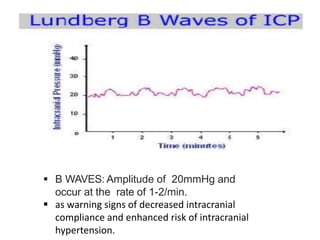
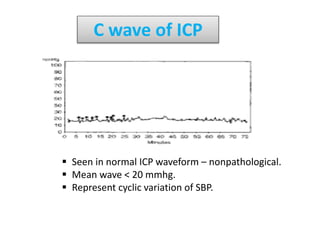
1. Intracranial pressure monitoring via an intraventricular catheter, which is the gold standard. Normal ICP is 10-15 mmHg in adults and waveforms can indicate increased ICP.
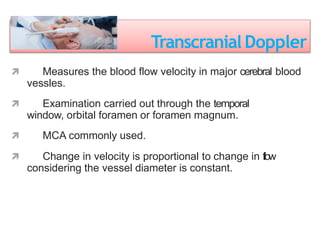
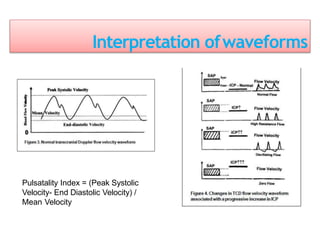
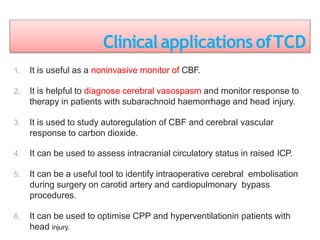
2. Transcranial Doppler measures blood flow velocity in cerebral arteries to monitor for vasospasm.
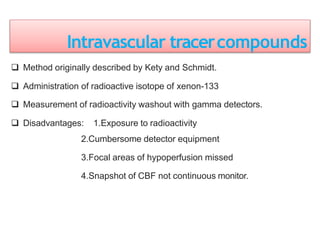
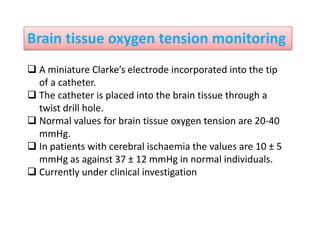
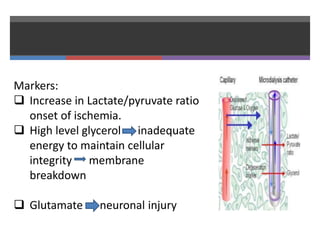
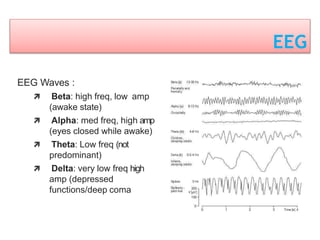
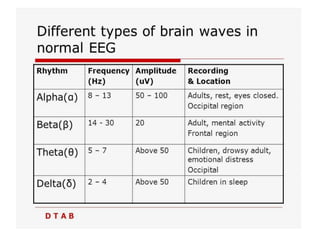
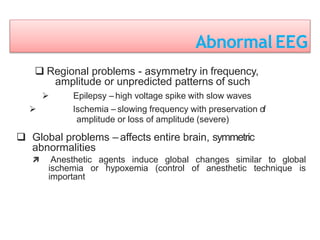
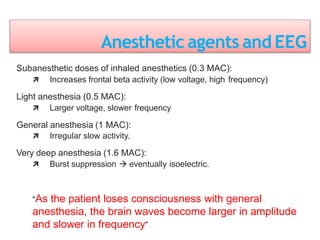
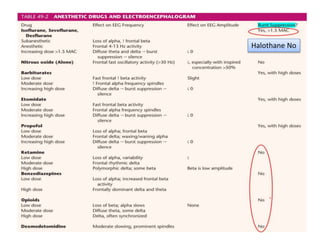
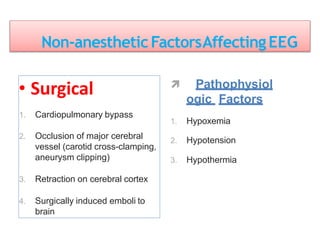
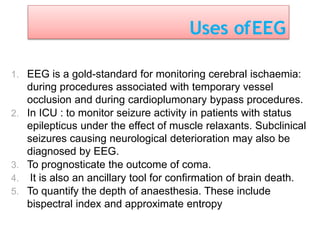
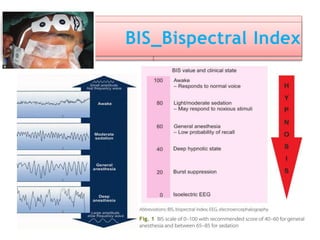
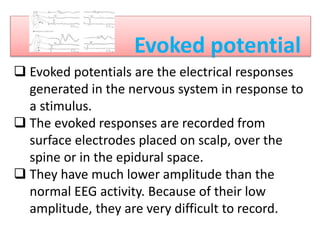
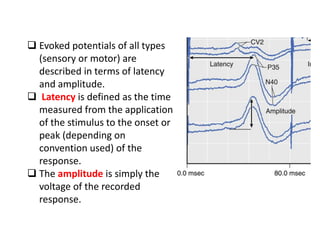
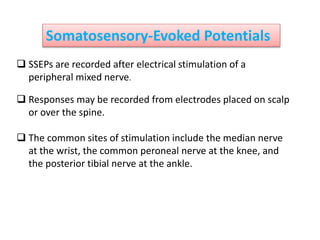
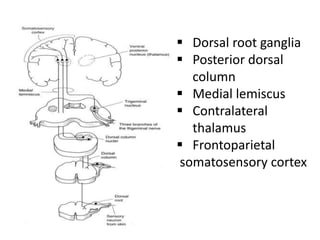
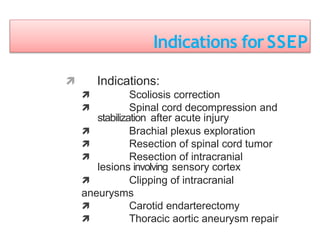
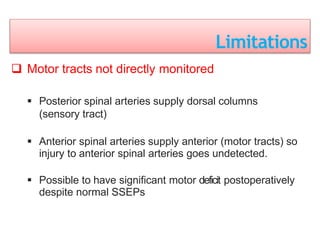
3. EEG, evoked potentials, brain tissue oxygen monitors, microdialysis, and near infrared spectroscopy provide additional insights into brain activity, oxygenation, and metabolism. EEG changes with anesthesia and can detect ischemia. Bispectral index monitors depth of anesthesia.