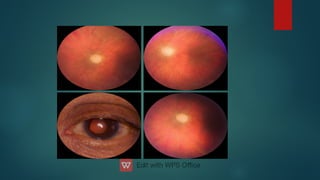
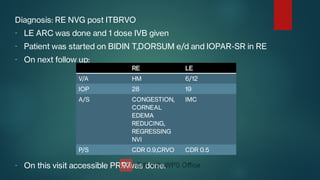
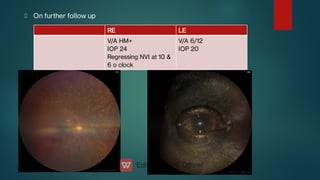
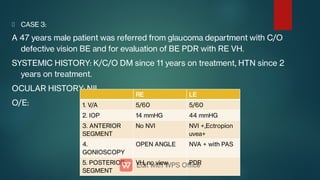
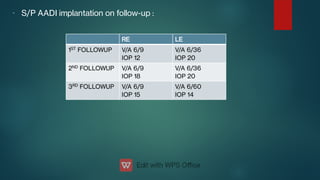
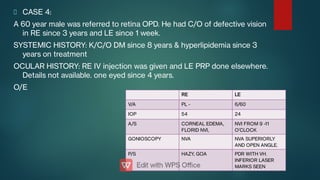
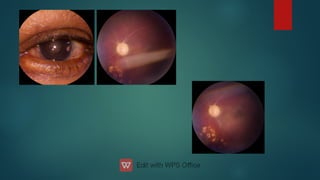
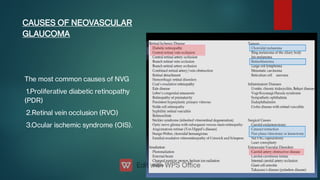
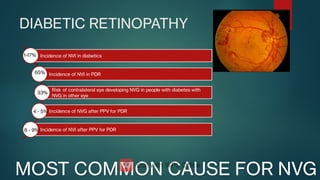
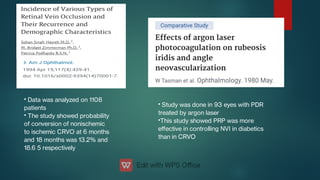
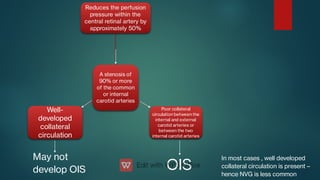
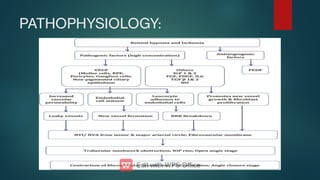
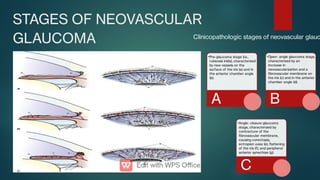
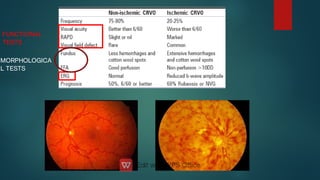
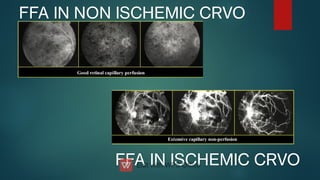
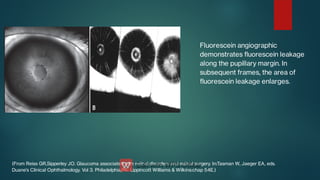
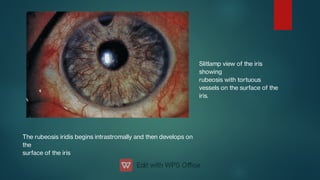
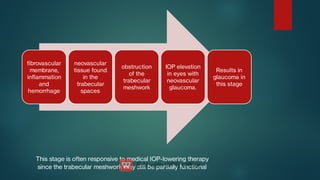
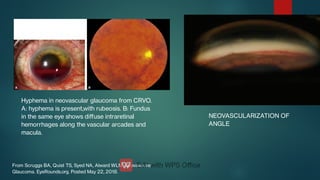
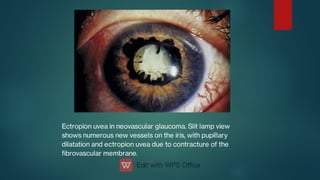
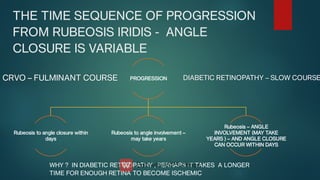
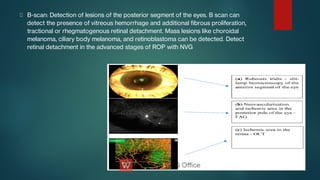
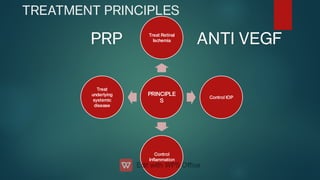
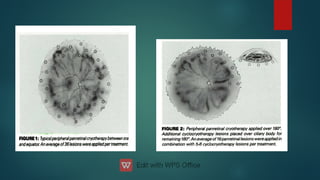
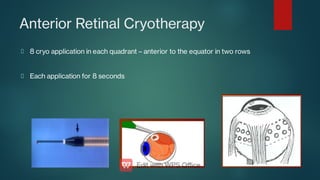
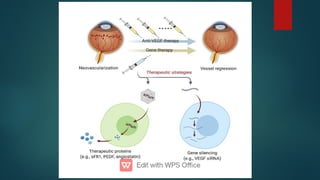
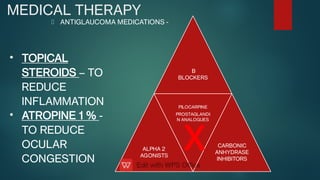
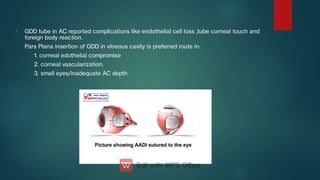
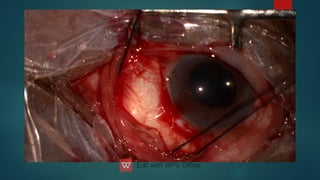
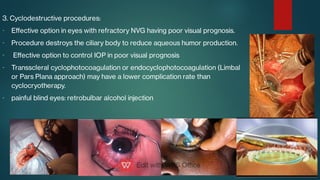
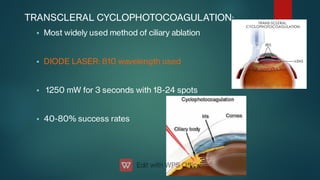
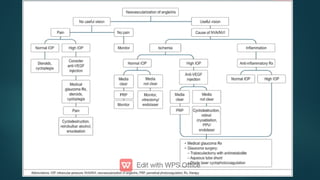
The document presents multiple cases of neovascular glaucoma (NVG) and discusses its diagnosis, management, and associated risk factors. Key causes of NVG identified include proliferative diabetic retinopathy, retinal vein occlusion, and ocular ischemic syndrome, with management often involving interventions like laser photocoagulation and intravitreal injections. The document also highlights the pathophysiology and clinical stages of NVG, emphasizing the importance of early detection and treatment to prevent severe vision impairment.