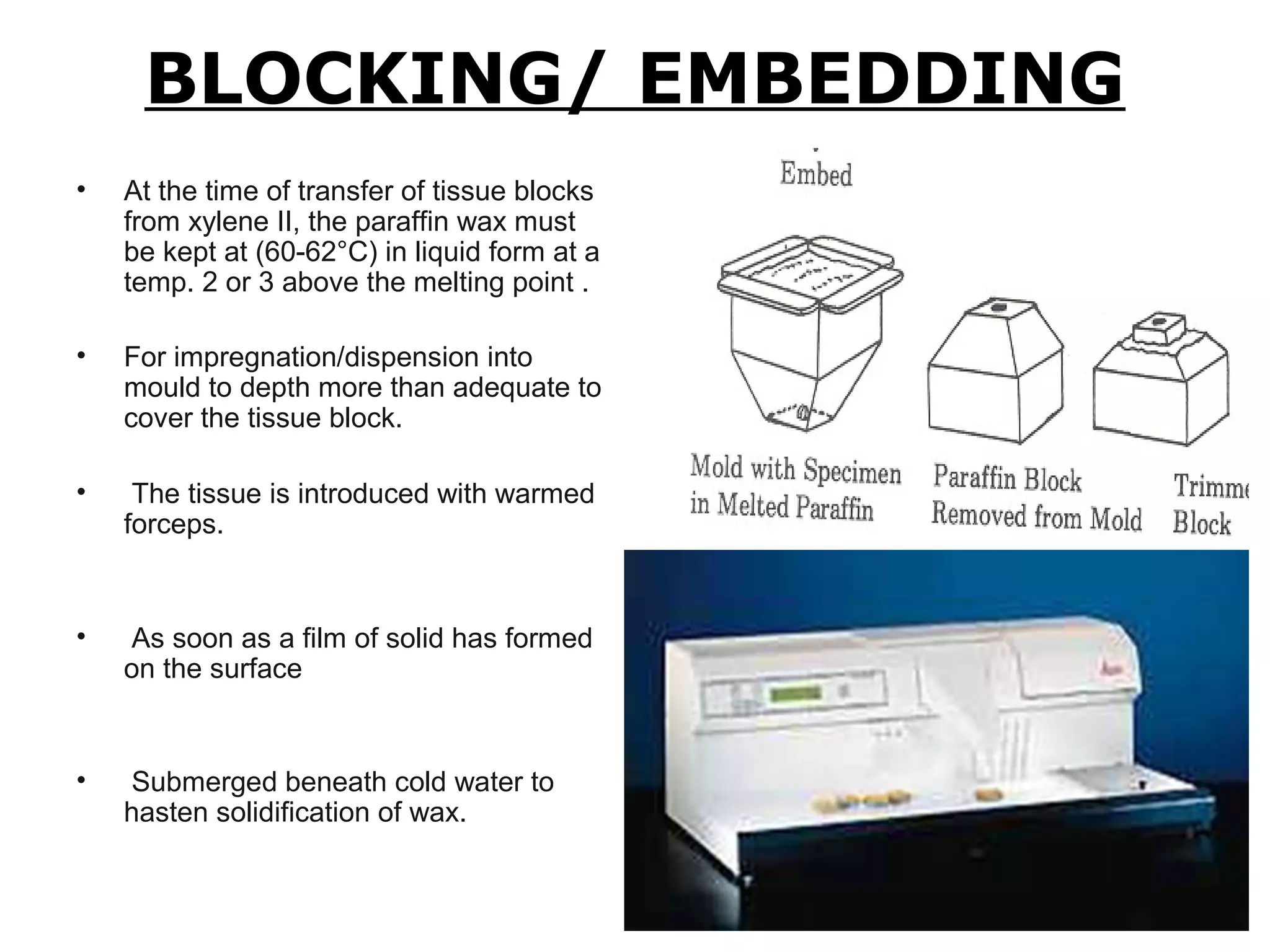
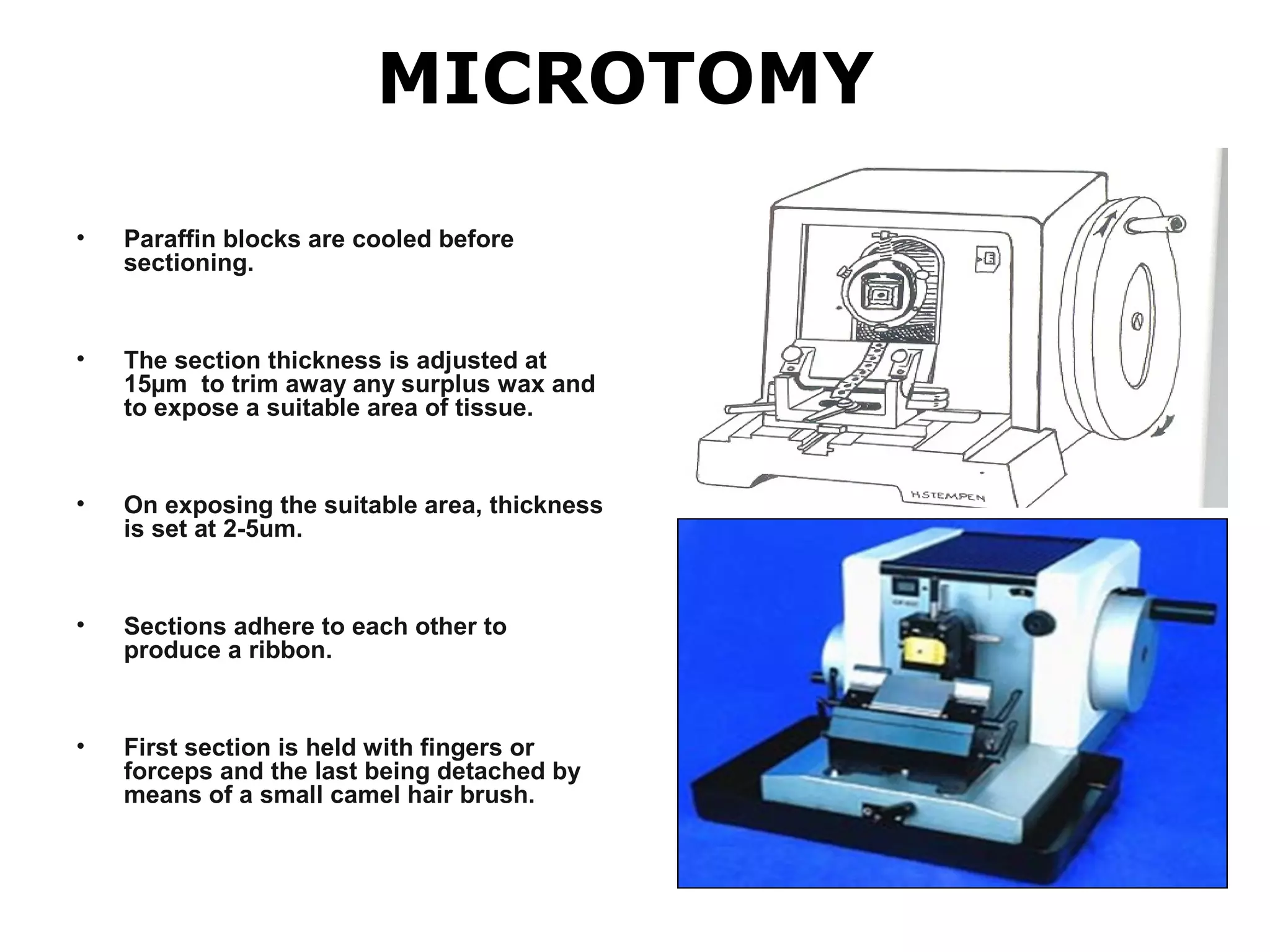
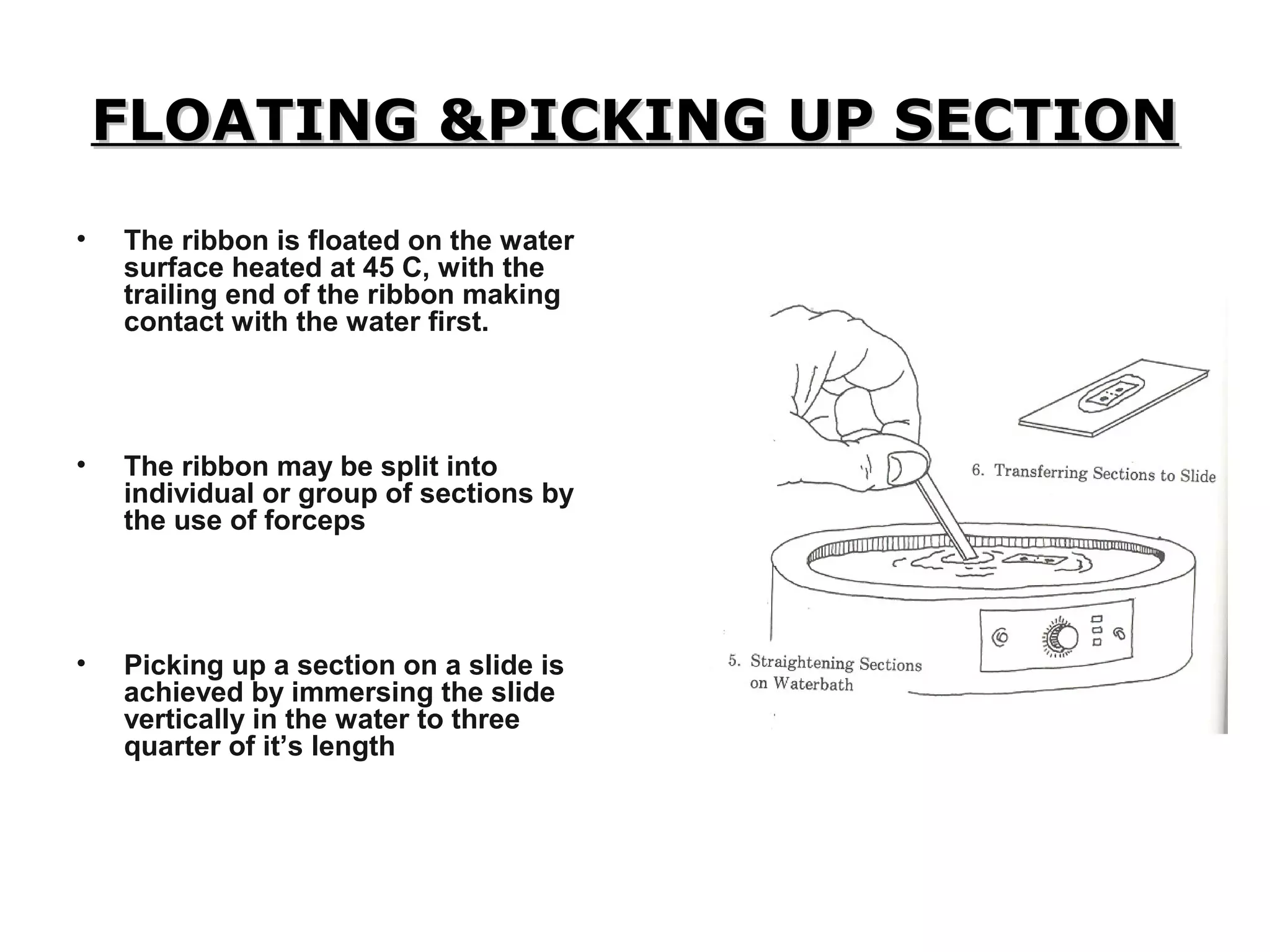
Histopathology techniques are used to demonstrate minute structural alterations in tissues caused by disease. Key techniques include fixing tissues in formalin to preserve structure, processing tissues through dehydration, clearing and infiltration steps, embedding in paraffin wax, sectioning with a microtome, staining, and mounting slides. Histopathology allows diagnosis of diseases through microscopic examination of tissue structures and any pathological changes present. It is a crucial technique when other testing may not be possible or provides definitive confirmation of diseases.