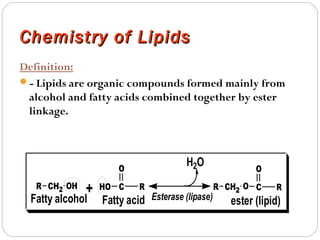
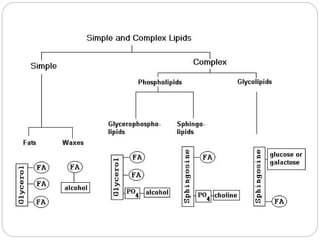
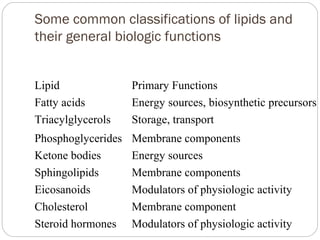
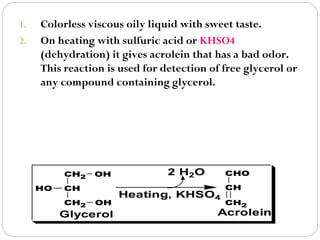
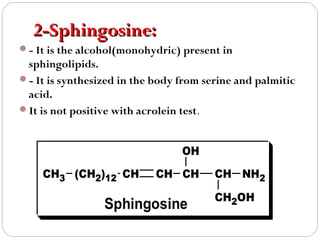
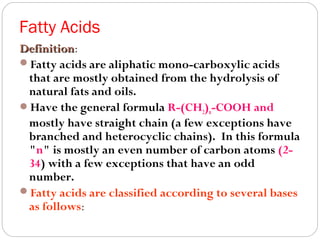
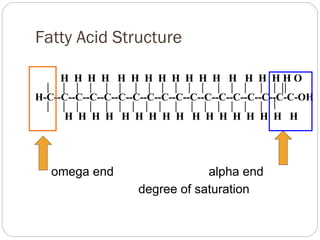
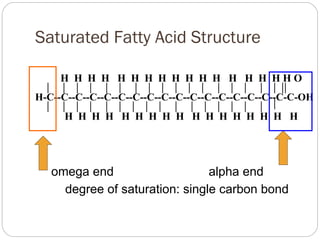
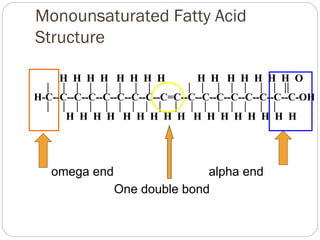
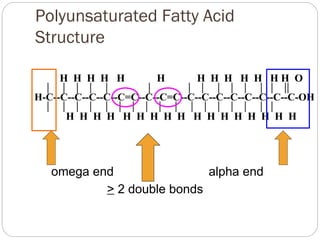
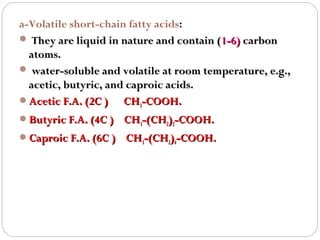
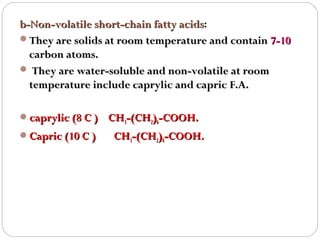
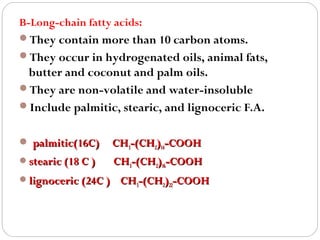
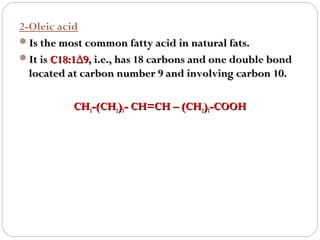
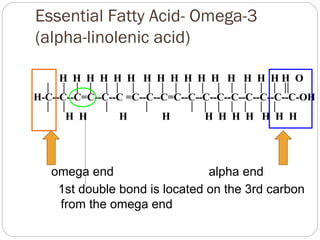
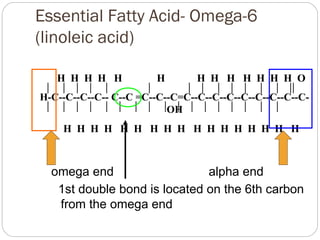
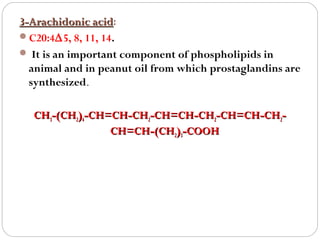
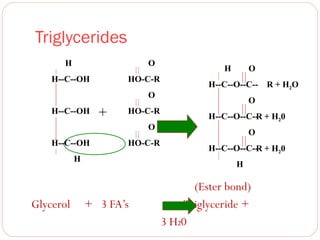
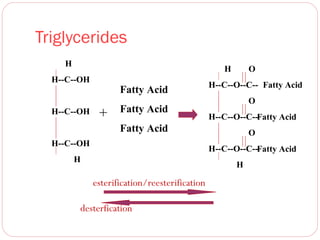
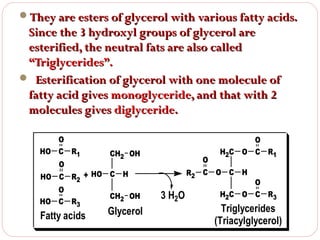
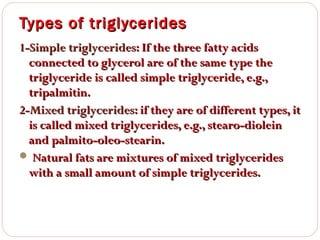
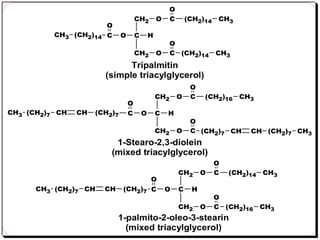
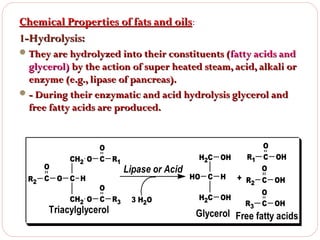
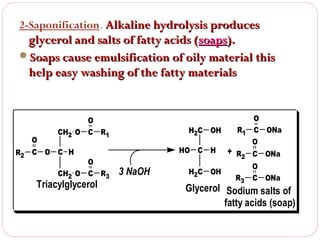
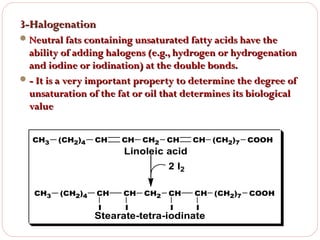
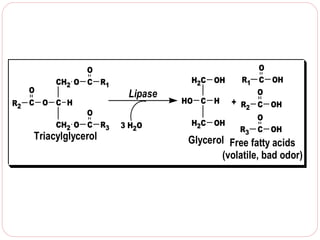
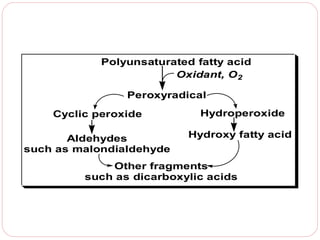
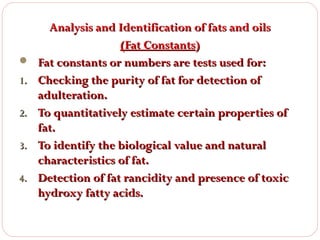
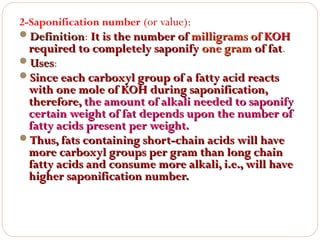
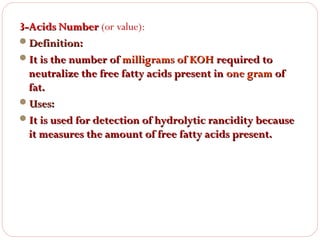
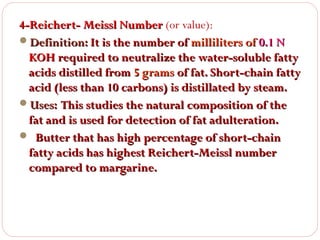
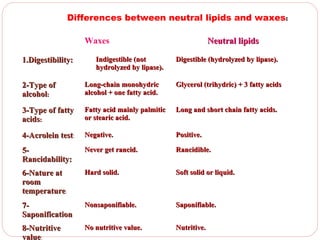
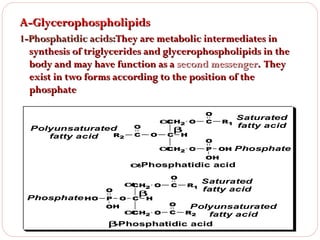
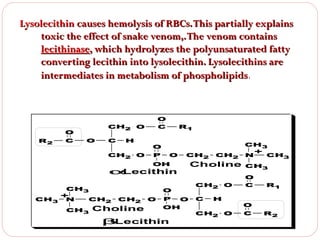
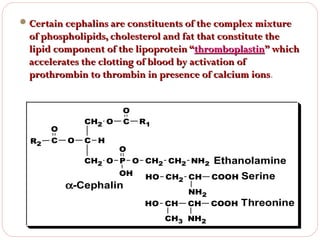
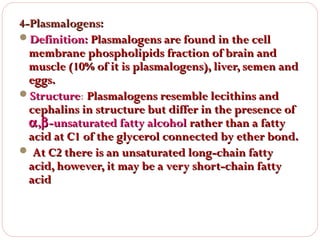
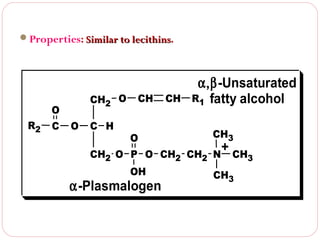
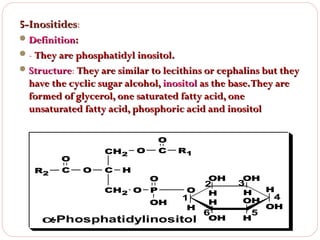
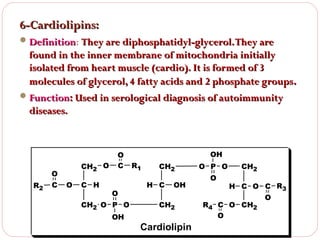
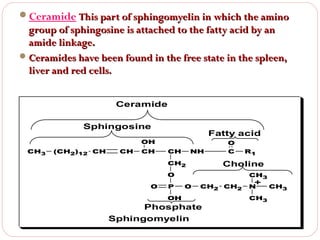
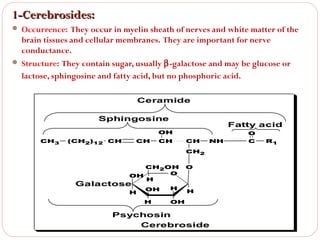
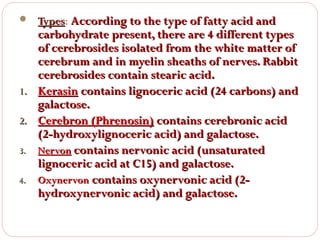
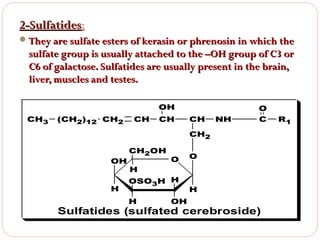
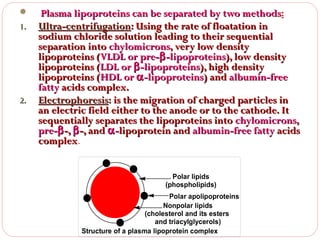
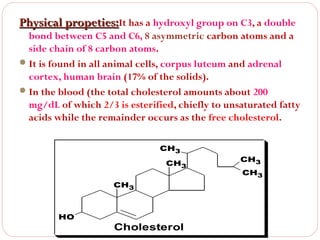
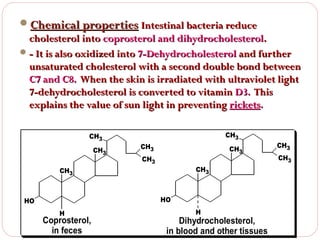
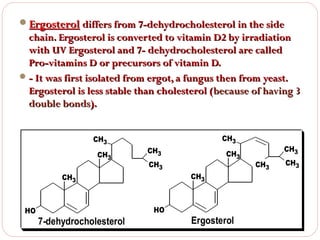
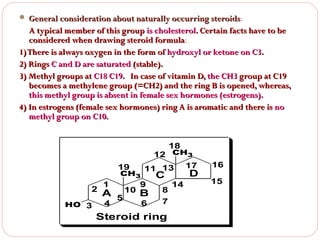
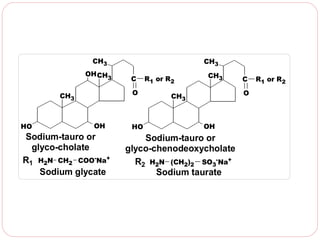
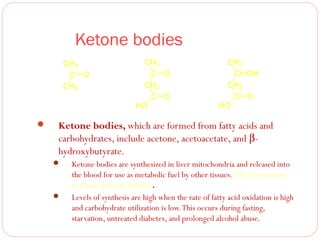
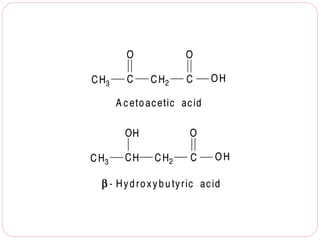
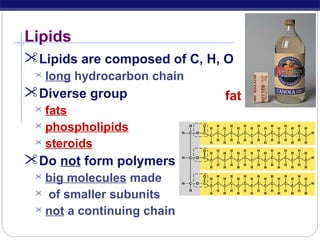
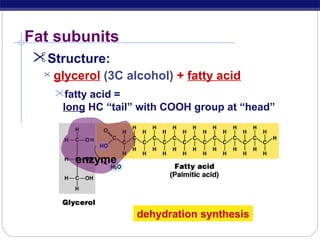
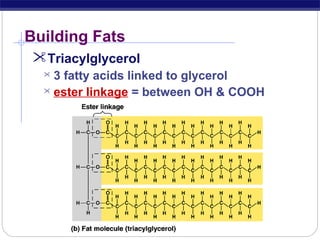
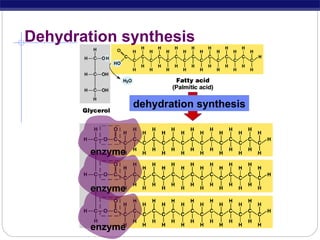
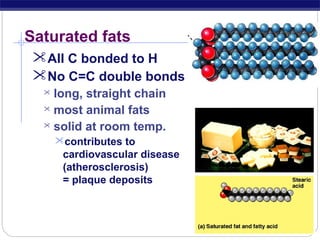
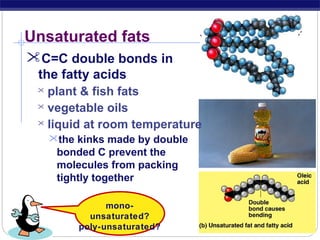
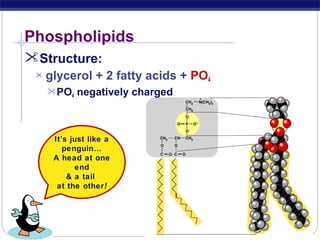
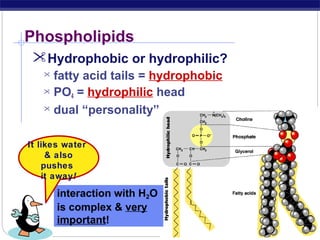
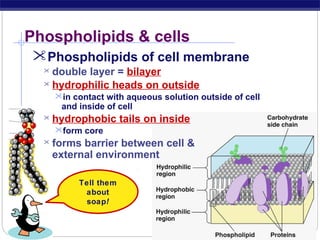
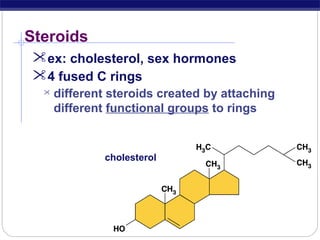
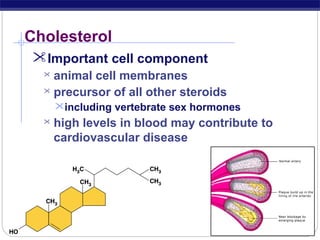
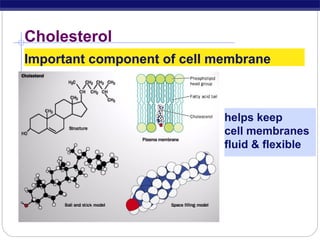
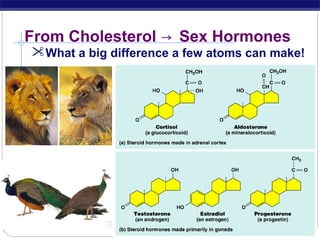
This document discusses lipids, which are organic compounds formed from alcohol and fatty acids combined by ester linkages. Lipids include fats, oils, waxes, and related compounds. They are insoluble in water but soluble in organic solvents. Triglycerides are the most abundant lipids, consisting of fatty acids esterified to a glycerol backbone. Triglycerides serve important functions like energy storage and insulation. Essential fatty acids must be obtained through diet as the human body cannot synthesize them and they are required for growth, metabolism, and physiological processes.