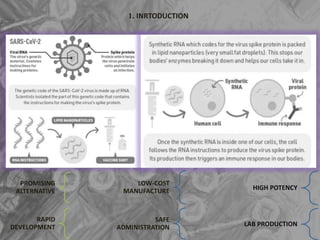
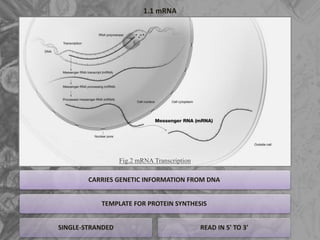
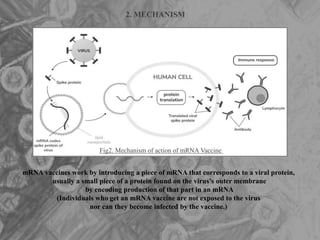
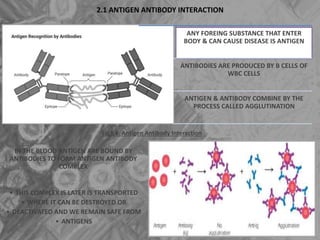
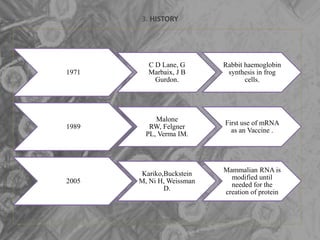
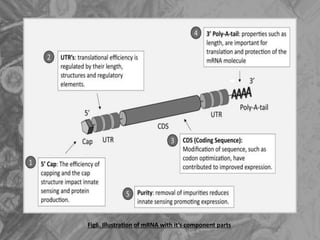
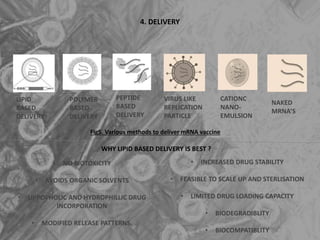
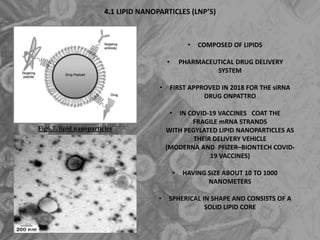
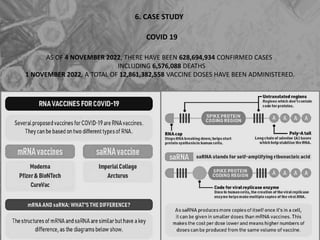
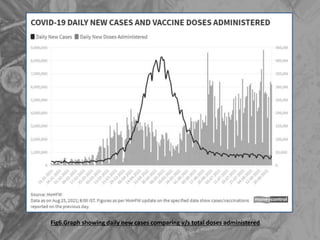
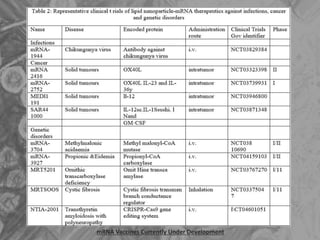
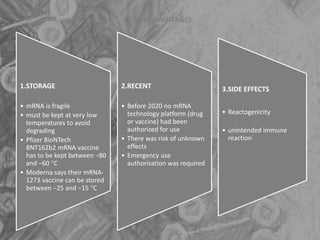
The document is a seminar presentation on mRNA vaccines, highlighting their mechanism, history, delivery methods, applications, advantages, and disadvantages. It details the role of lipid nanoparticles in the delivery of mRNA vaccines and discusses their potential in rapid vaccine development for pandemics like COVID-19. The seminar concludes with the recognition of mRNA vaccines as a groundbreaking advancement in vaccinology, with ongoing research indicating their future potential in treating other diseases.