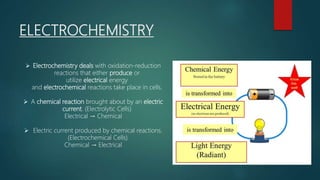
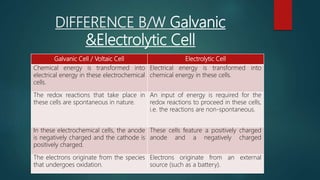
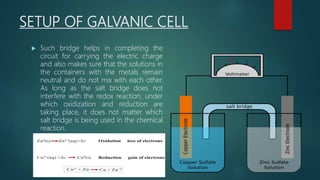
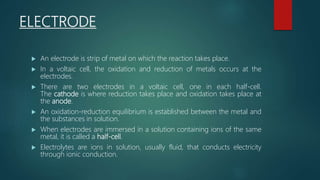
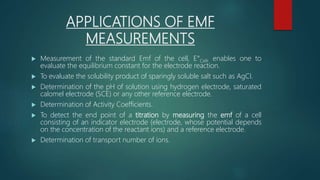
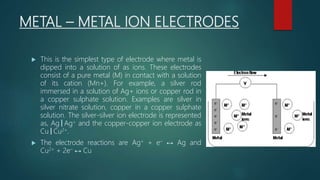
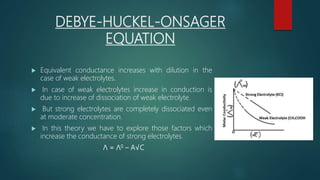
This document provides information about electrochemistry. It discusses how electrochemistry deals with oxidation-reduction reactions that produce or utilize electrical energy. There are two main types of electrochemical cells: galvanic cells which convert chemical energy to electrical energy, and electrolytic cells which use electrical energy to drive non-spontaneous chemical reactions. The document explains the basic setup and workings of these two cell types.





































![NERNST EQUATION
The Nernst equation provides a relation between the cell potential of an
electrochemical cell, the standard cell potential, temperature, and the reaction
quotient.
Even under non-standard conditions, the cell potentials of electrochemical cells can be
determined with the help of the Nernst equation.
The equation was introduced by a German chemist named Walther Hermann Nernst.
Ecell = E0 – [RT/nF] ln Q
Ecell = cell potential of the cell
E0 = cell potential under standard conditions
R = universal gas constant
T = temperature
n = number of electrons transferred in the redox reaction
F = Faraday constant
Q = reaction quotient](https://image.slidesharecdn.com/mphilelectrochemistry-210531160349/85/Mphil-electrochemistry-45-320.jpg)





