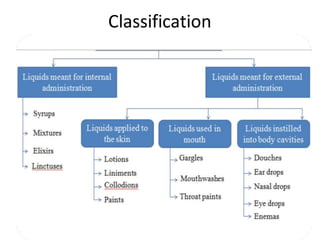
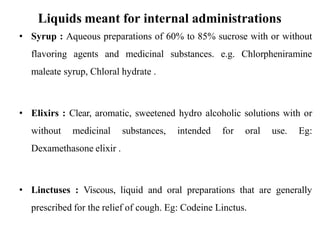
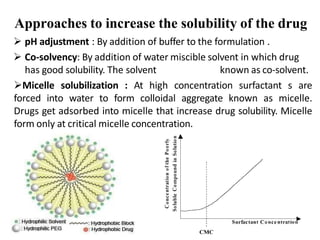
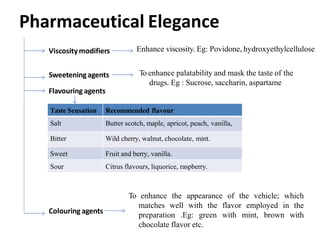
This document discusses monophasic liquid dosage forms, including their advantages and disadvantages. It covers classification of monophasic liquids for internal and external administration. Key considerations for formulation include solubility, stability, preservatives, and pharmaceutical elegance. Manufacturing considerations include raw materials, equipment used, and cleaning procedures. Recent advances discussed are methods to enhance drug solubility like nanocrystals and delivery systems like novel parenteral and ophthalmic drug delivery.