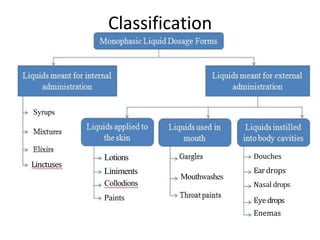
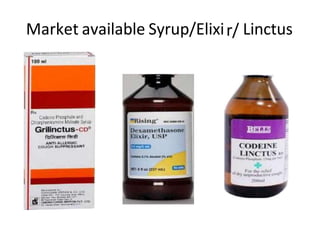
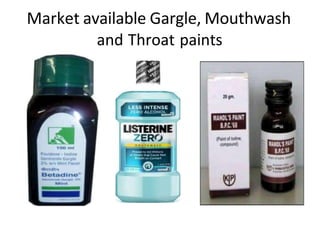
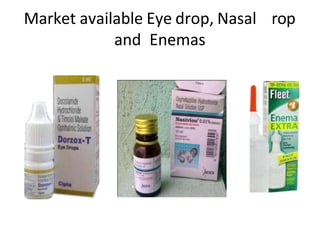
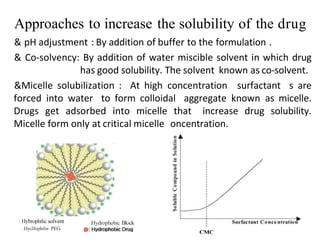
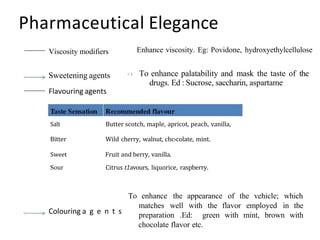
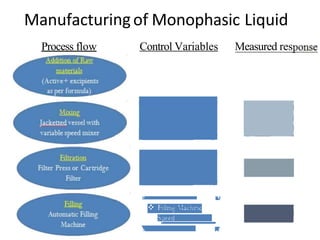
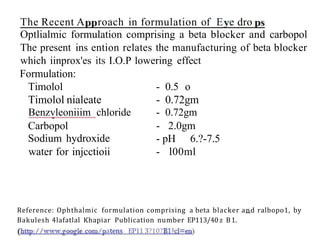
This document discusses monophasic liquid dosage forms. It begins with an introduction defining monophasic liquids as solutions containing two or more components in a single liquid phase. It then covers classifications of monophasic liquids including syrups, elixirs, and linctuses. The document discusses formulation considerations like solubility, stability, and preservatives. It also covers manufacturing considerations and recent advances in monophasic liquid formulations including nanocrystals and novel parenteral delivery approaches.