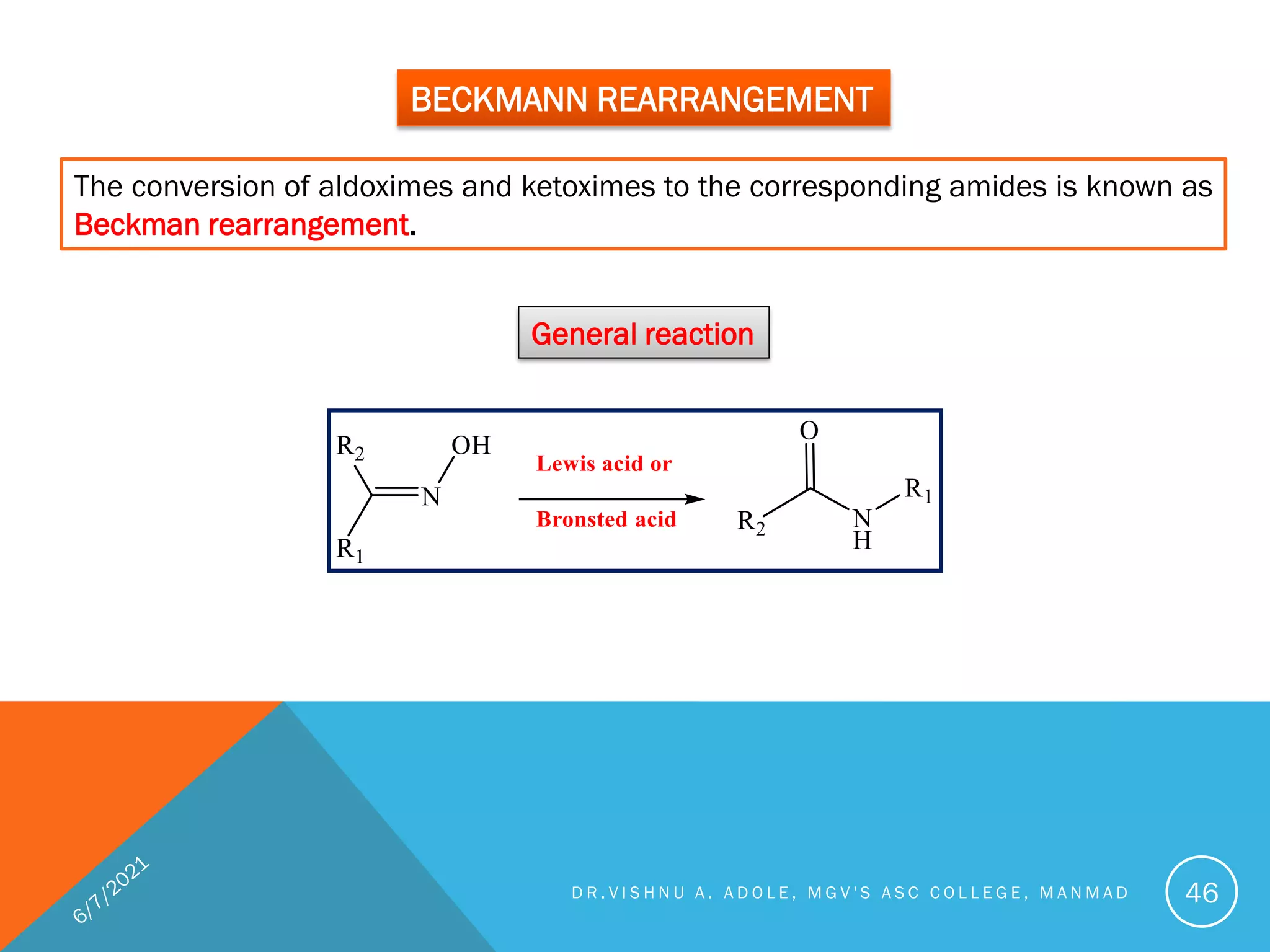
This document discusses various rearrangement reactions including pinacol-pinacolone rearrangement, Favorskii rearrangement, Curtius rearrangement, Beckman rearrangement, and others. It provides mechanisms, requirements, and examples for each reaction. Crossover experiments are described as a way to determine if a reaction proceeds via an intramolecular or intermolecular pathway. Stereochemistry and migratory aptitude are also addressed for several reactions.









![PINACOL – PINACOLONE REARRANGEMENT
The acid catalyzed transformation of Vicinal diols [1, 2 diols] to ketones or
aldehydes is known as Pinacol - Pinacolone Rearrangement.
General reaction
D R . V I S H N U A . A D O L E , M G V ' S A S C C O L L E G E , M A N M A D 10](https://image.slidesharecdn.com/rearrangementreactionstybsc-dr-210607064144/75/Rearrangement-reactions-10-2048.jpg)





















































![The thermal [3,3]-sigmatropic rearrangement of allyl-vinyl ethers to the corresponding
γ,δ-unsaturated carbonyl compound is called Claisen rearrangement. When allyl phenyl
ethers are used as reactants, the rearrangement is called as aromatic Claisen
rearrangement.
CLAISEN REARRANGEMENT
General reaction
Aliphatic Claisen rearrangement Aliphatic Claisen rearrangement
Allyl vinyl
ether
g,d-
unstaurated
aldehyde
Allyl phenyl
ether
o-Allyl phenol
D R . V I S H N U A . A D O L E , M G V ' S A S C C O L L E G E , M A N M A D 65](https://image.slidesharecdn.com/rearrangementreactionstybsc-dr-210607064144/75/Rearrangement-reactions-65-2048.jpg)



