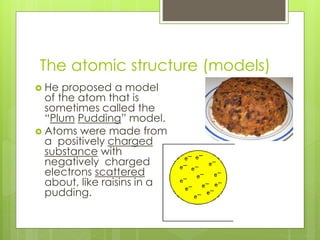
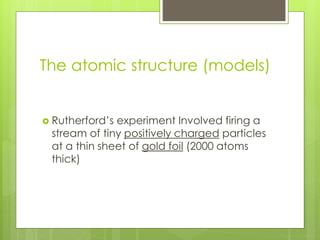
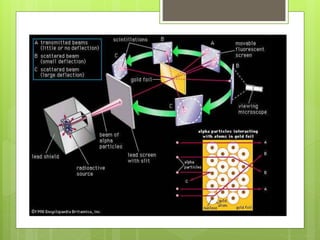
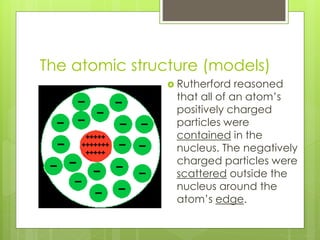
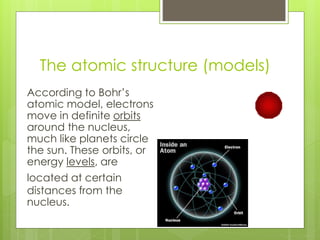
The document discusses the evolution of atomic models from Democritus' early theory of indivisible 'atomos' to modern concepts. It highlights key contributions from scientists such as John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr in understanding atomic structure. Throughout history, the atomic model has transformed significantly as new discoveries were made, each building on the previous knowledge.