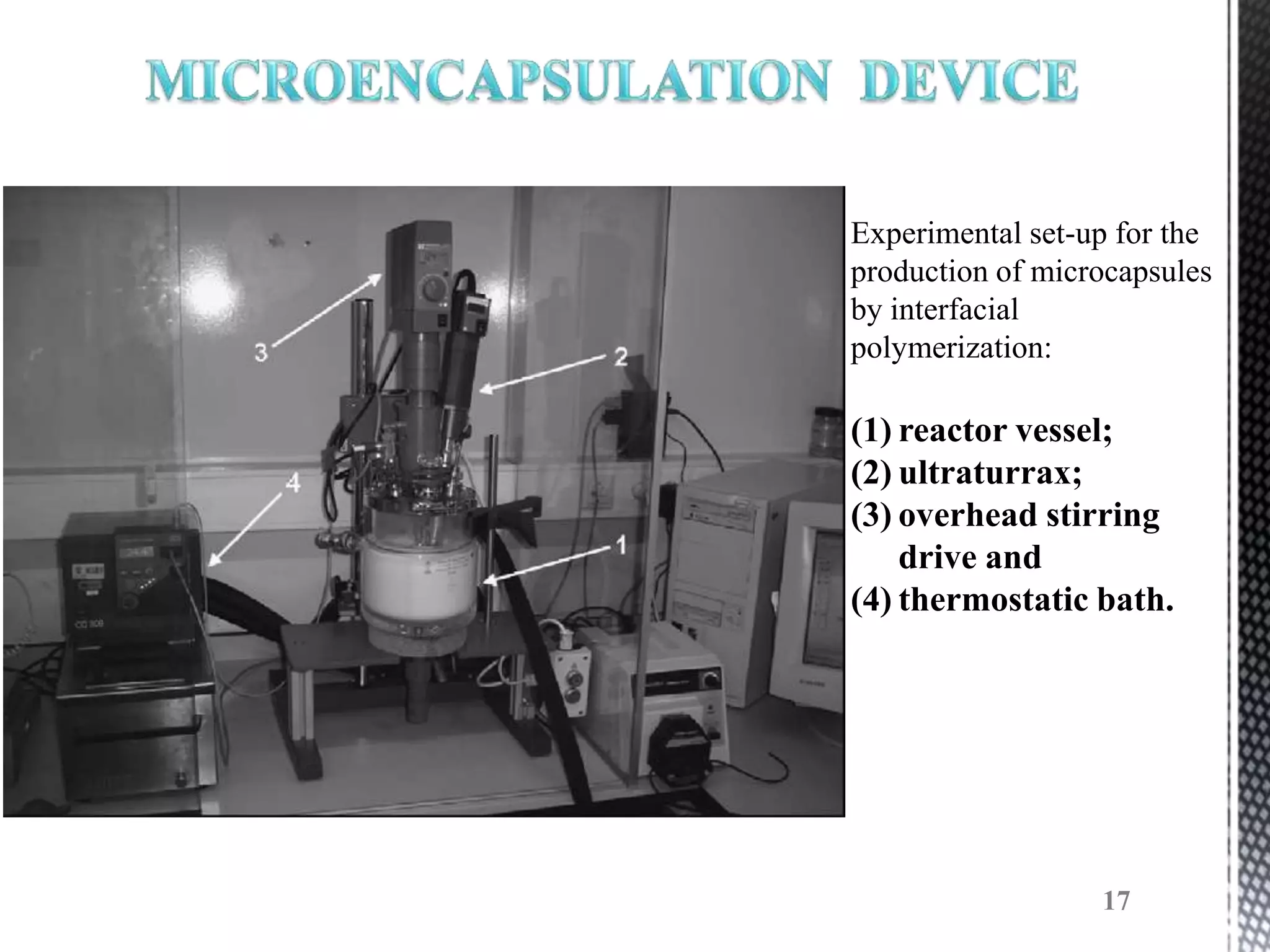
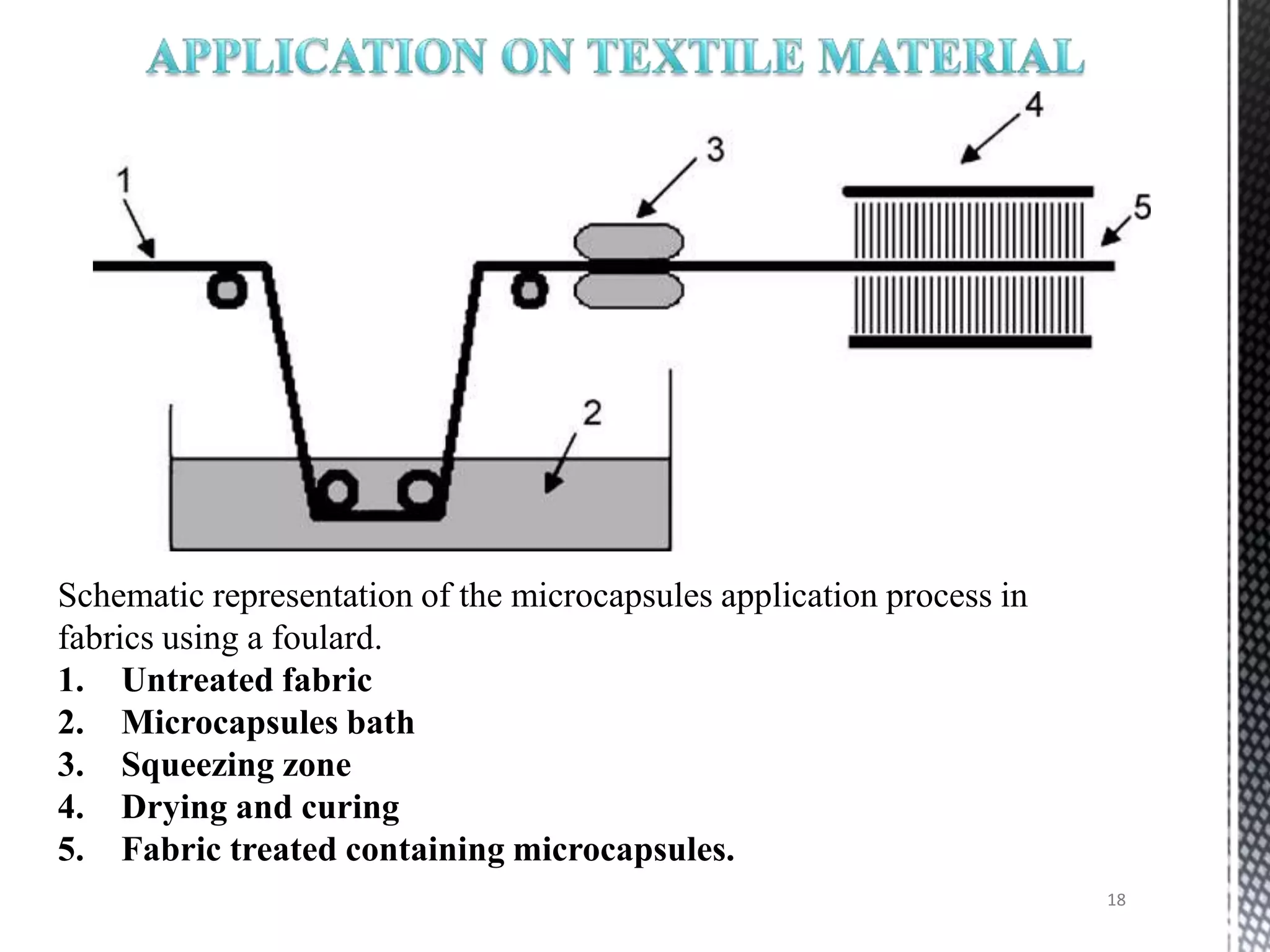
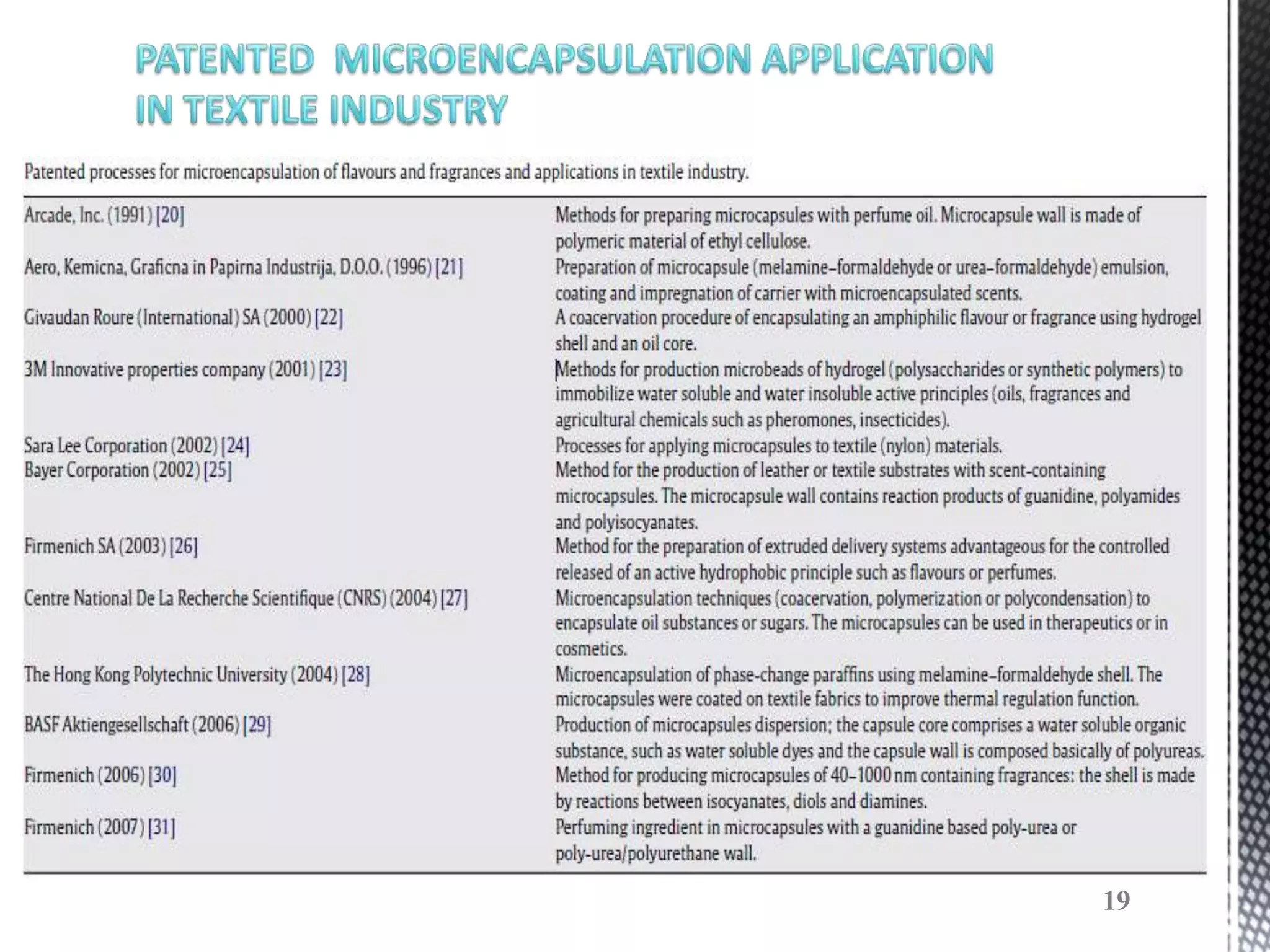
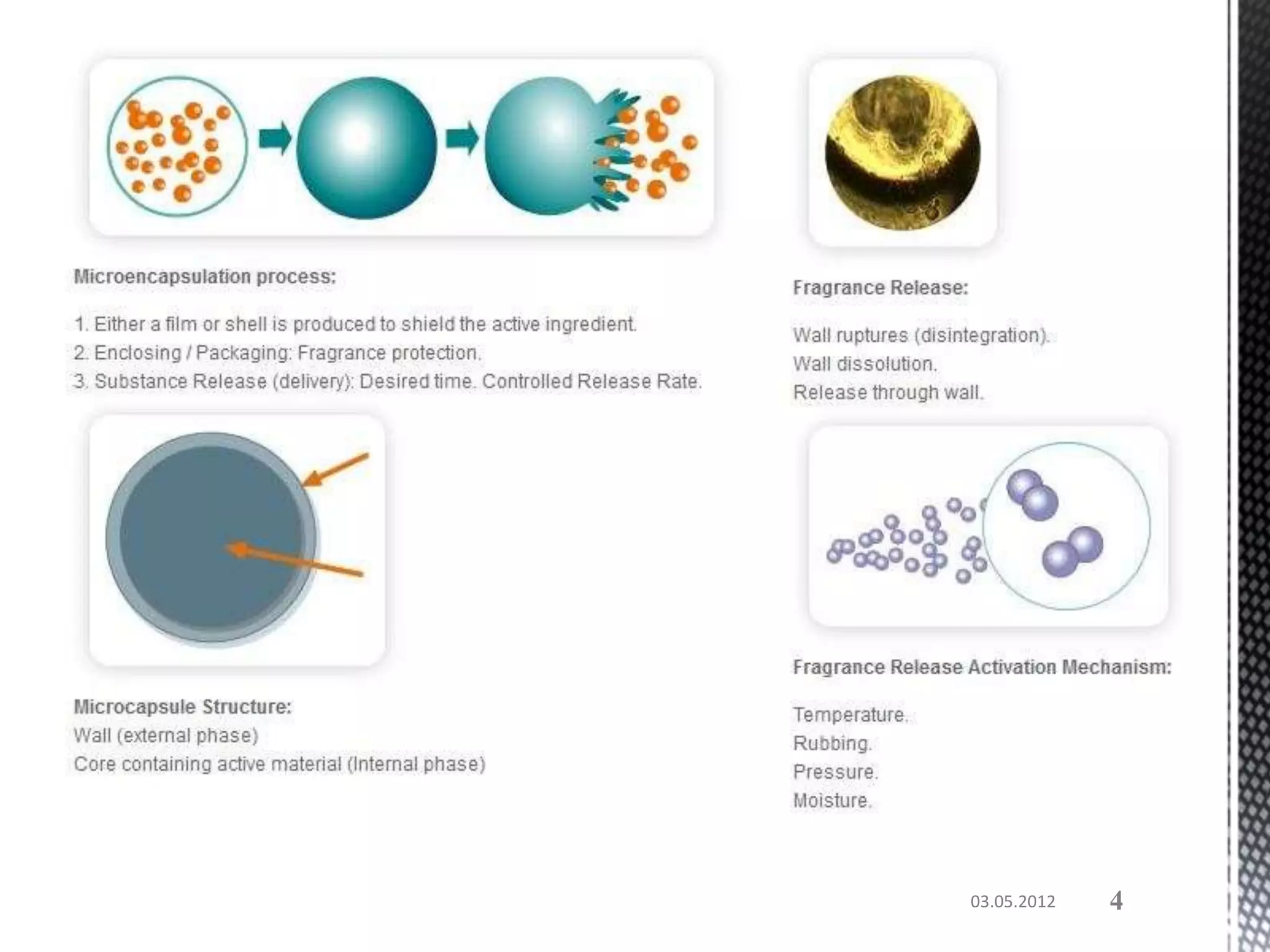
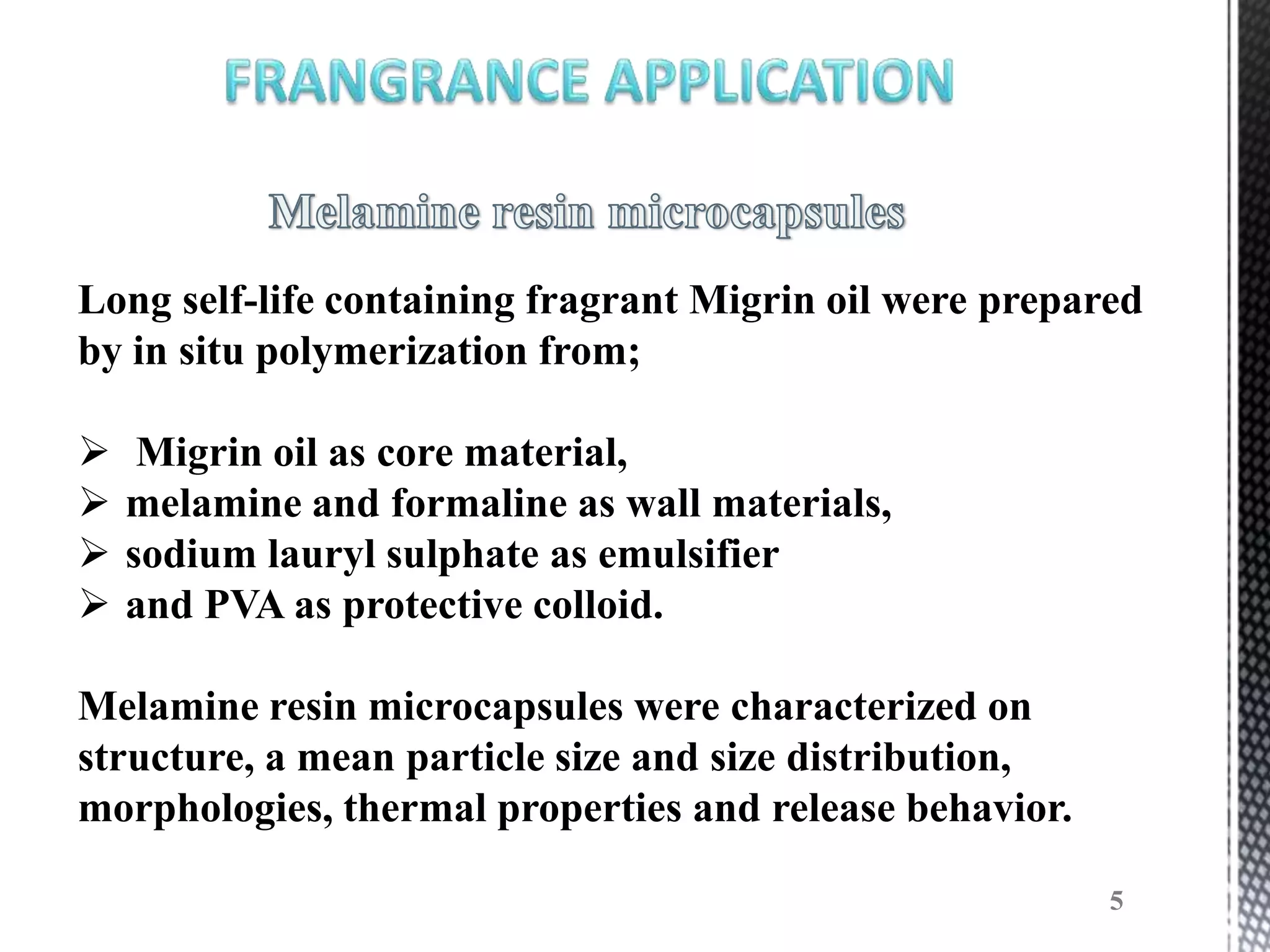
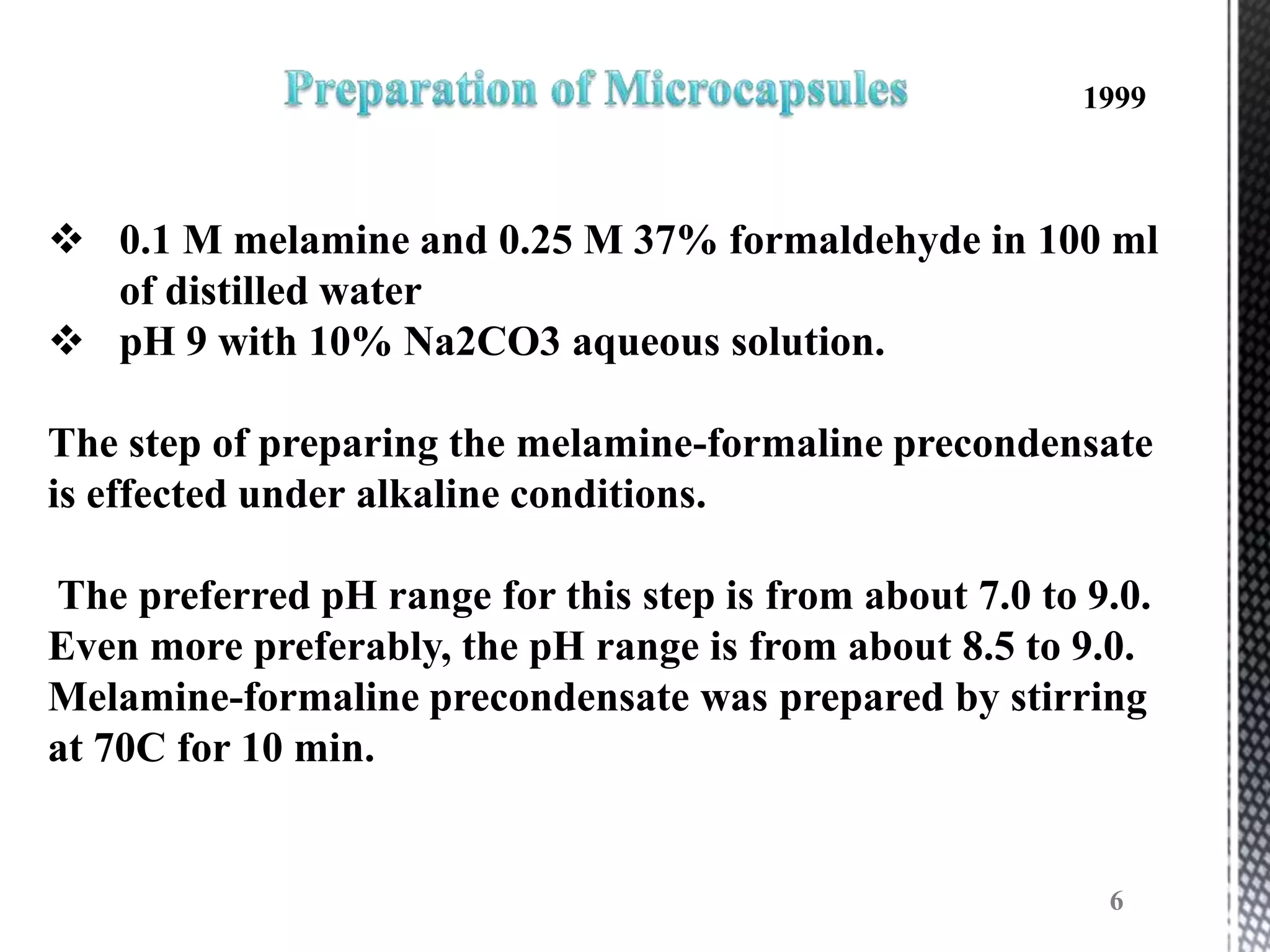
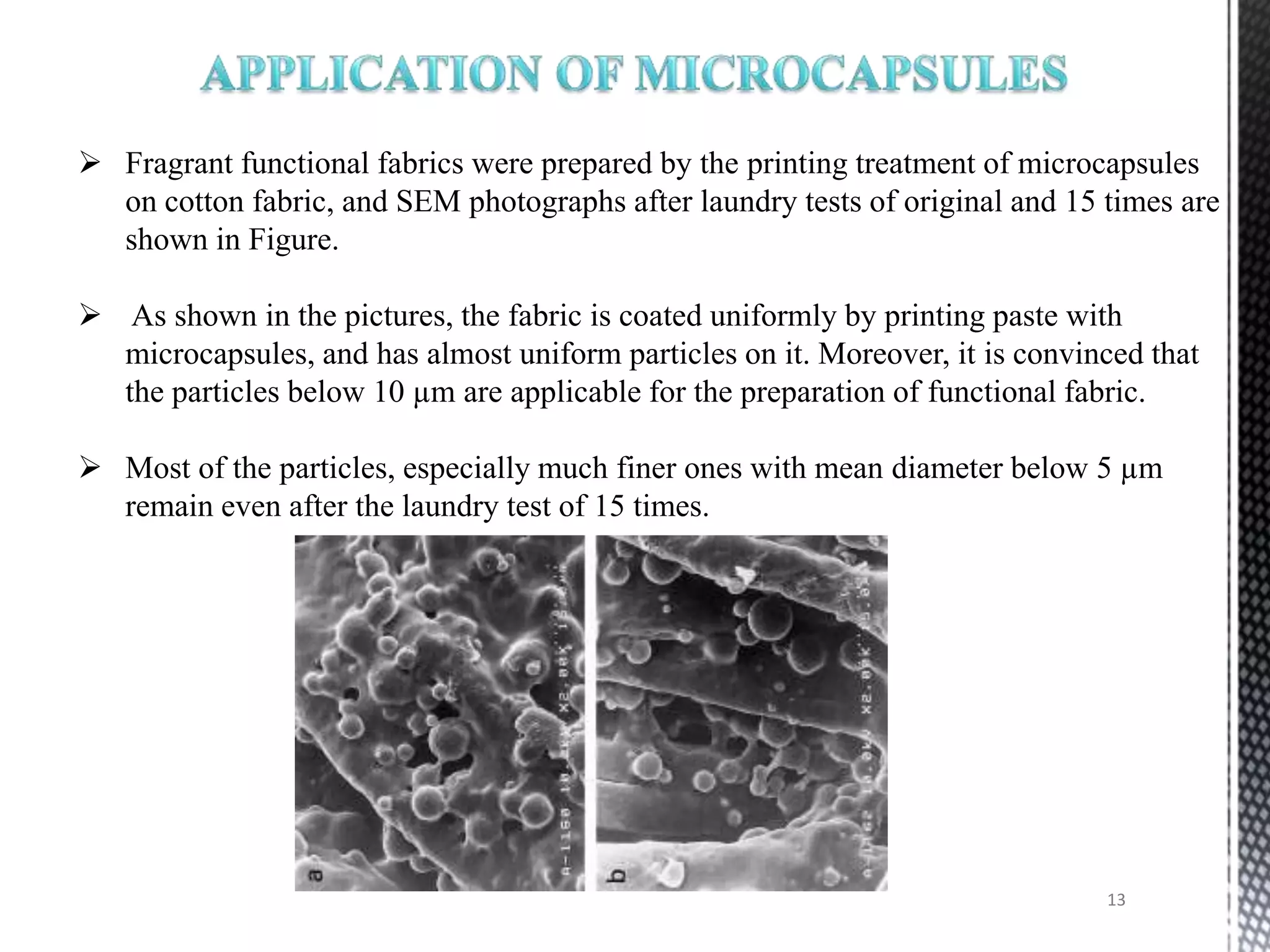
The document discusses microencapsulation techniques for imparting long-lasting fragrances to textiles. It describes how microencapsulation allows fragrances to remain on fabrics for more wash cycles compared to direct application. Various microencapsulation methods are examined, including using melamine resin and poly(l-lactide) to encapsulate fragrant oils. Scanning electron microscope images show microcapsules adhered to fabrics after laundry tests. The document also explores producing polyurethane microcapsules containing perfumes via interfacial polymerization and applying them to fabrics with a foulard.




![The materials used for the formulation of perfume were:
Limonene (lemon scent – LMN) (Sigma–Aldrich),
Methyl cedryl ketone (vetiver scent – MCK) (Sigma–Aldrich),
Methyl dihydrojasmonate (jasmine scent – MJD) (Sigma–Aldrich)
And 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylcyclopenta[g]-2-
enzopyran solution (galaxolide); 50% in diethyl phthalate (dep)
(musk scent) (Sigma–Aldrich).
REACTANTS
Hexamethylene-1,6-diisocyanate (HMDI) (Bayer, Desmodur W) as the
isocyanate;
Dibutyltin dilaurate (DBDTL) (Sigma–Aldrich) as the catalyst;
Polyethylene glycol 400 (PEG 400) (Sigma–Aldrich) as the polyol;
Ethylenediamine (EDA) (Panreac) as amine I;
Hydrazine monohydrate (HYD) (Sigma–Aldrich) as amine II;
Polyvinyl alcohol (PVA) (Celanese Chemicals, Celvol 840) as protective
colloid
Triton CA (Dow Company) as emulsifier. 03.05.2012 14](https://image.slidesharecdn.com/sinanzer-te428-120503204142-phpapp01/75/MICROENCAPSULATION-FRAGRANCE-APP-14-2048.jpg)