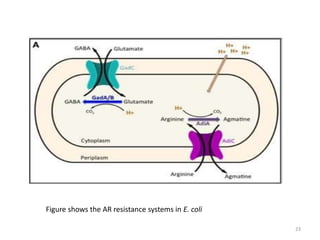
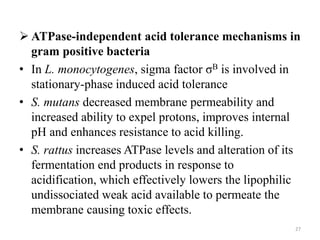
This document summarizes microbial responses to acid stress. It discusses how bacteria like E. coli, Salmonella, and Helicobacter pylori have developed mechanisms to regulate internal pH and withstand acidic environments. These include proton pumps, amino acid decarboxylases, and the urease enzyme system. Regulatory systems like sigma factors and two-component systems help induce acid shock proteins and responses during acid exposure. Gram-positive bacteria like streptococci take a more flexible approach by allowing internal pH to decrease along with external pH.