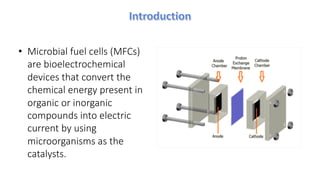
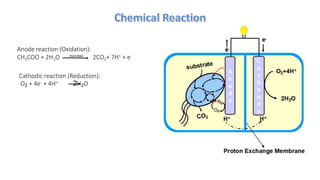
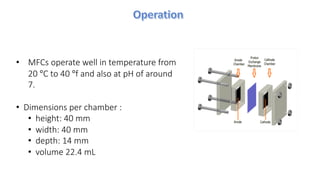
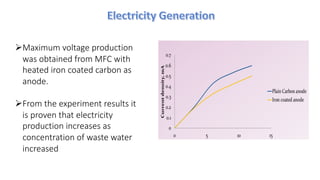
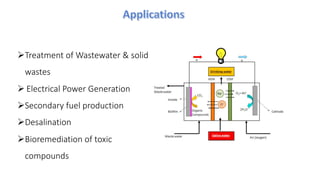
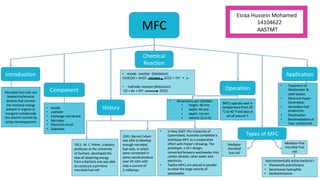
Microbial fuel cells (MFCs) are bioelectrochemical devices that convert chemical energy from organic compounds into electricity using microorganisms. MFCs operate between 20-40°C and pH 7 using bacteria like Shewanella putrefaciens and Geobacteraceae to catalyze the anode and cathode reactions. The history of MFCs dates back to 1911 with early prototypes, while the University of Queensland developed a 10L prototype in 2007 to generate electricity from brewery wastewater. MFCs can be used to treat wastewater and produce power, hydrogen, or desalinated water while remediating toxins.