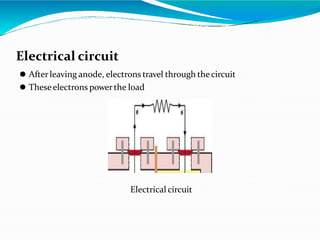
This document provides an overview of microbial fuel cells (MFCs). MFCs use bacteria to convert the chemical energy in organic and inorganic matter into electricity. The document describes the components of an MFC, including the anode where bacteria live and degrade substrate, producing electrons and protons, a cathode where oxygen is reduced, and a proton exchange membrane. It explains that protons flow through the membrane while electrons flow an external circuit, powering a load. The document also discusses applications like wastewater treatment and power generation, advantages such as producing renewable energy from waste, and limitations such as low power densities.