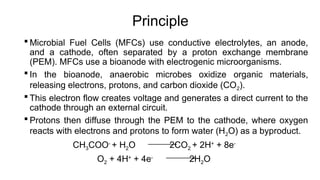
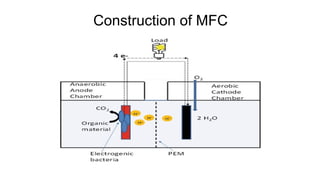
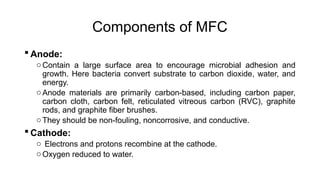
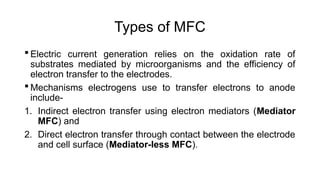
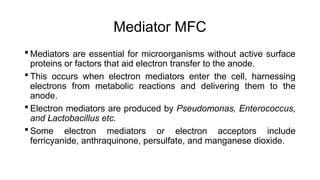
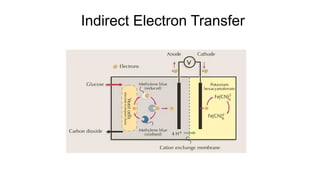
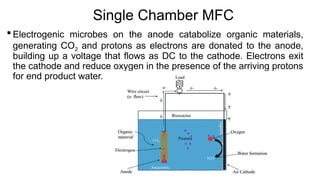
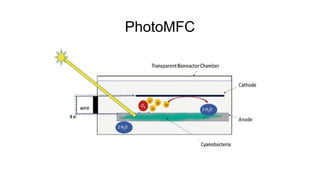
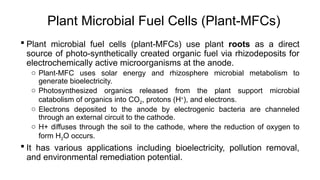
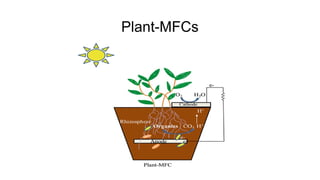
Bioelectricity involves electrical currents generated by living organisms and is utilized in bioelectrochemical systems (BESS) such as microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) for renewable energy applications. MFCs convert organic matter into electricity using bacteria, significantly cheaper than traditional fuel cells, and advance their efficiency with developments like mediator-less systems. Challenges for MFCs include slow start-up and low power output, but they have applications in waste treatment, power generation, and environmental remediation.