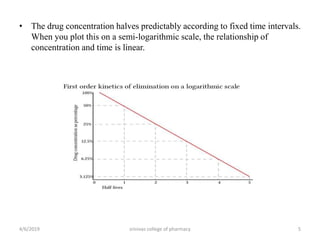
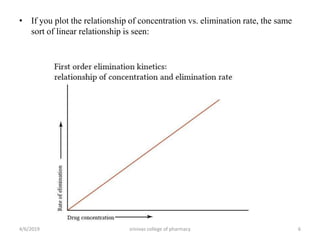
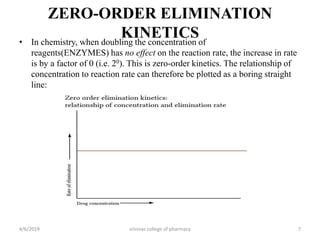
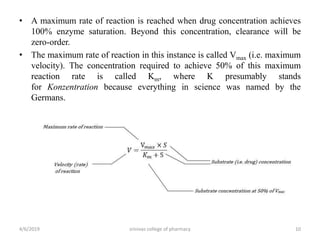
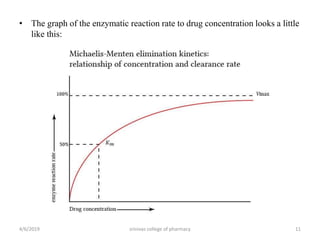
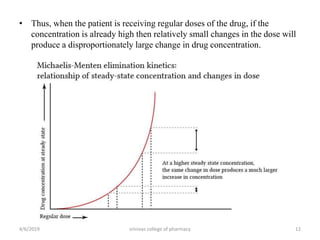
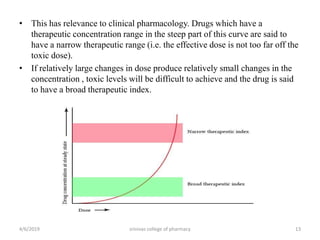
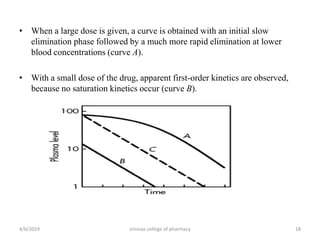
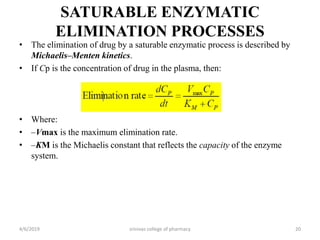
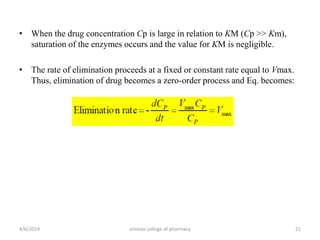
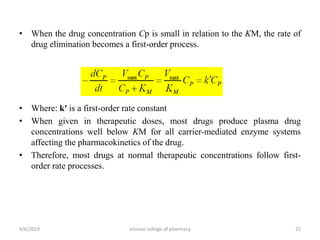
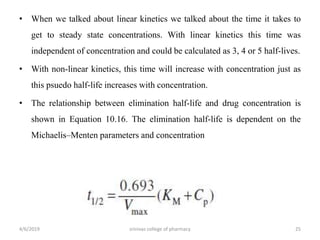
This document discusses saturation kinetics and nonlinear pharmacokinetics. It explains that drug clearance follows first-order kinetics at low concentrations but can become saturated and shift to zero-order kinetics at high concentrations due to limited enzyme capacity. This nonlinear behavior is described by Michaelis-Menten kinetics. A few drugs like phenytoin exhibit saturation kinetics in the therapeutic range, making their dosing more complex due to changing half-lives with concentration. Understanding saturation and nonlinear pharmacokinetics is important for safely dosing drugs that exhibit these behaviors.